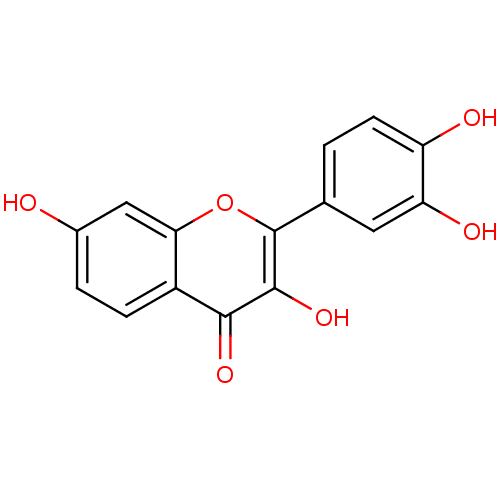
BDBM7457 2-(3,4-dihydroxyphenyl)-3,7-dihydroxy-4H-chromen-4-one::2-(3,4-dihydroxyphenyl)-3,7-dihydroxy-chromen-4-one::2-(3,4-dihydroxyphenyl)-3,7-dihydroxy-chromone;dihydrate::CHEMBL31574::Fisetin (14)::Fisetin, 15::fisetin
SMILES c1cc(c(cc1C2=C(C(=O)c3ccc(cc3O2)O)O)O)O
InChI Key InChIKey=XHEFDIBZLJXQHF-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 49 hits for monomerid = 7457
Found 49 hits for monomerid = 7457
Affinity DataIC50: 12nMAssay Description:Inhibition of AChE (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 350nMAssay Description:Inhibition of rat liver CK2 phosphorylation using RRRADDSDDDDD as substrate in presence of [32p]-ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 350nMAssay Description:Inhibition of human recombinant CK2 expressed in Escherichia coli using RRRADDSDDDDD as substrate after 10 mins in presence of [gamma-32P]ATPMore data for this Ligand-Target Pair
Affinity DataKi: 350nMAssay Description:Inhibition of CK2alpha (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 420nMT: 2°CAssay Description:Kinase activities were assayed in buffers containing substrate, enzyme, and inhibitor at 30 °C in the presence of 15 uM ATP/ [gamma-32P] ATP. 32...More data for this Ligand-Target Pair
Affinity DataIC50: 570nMpH: 7.0 T: 2°CAssay Description:Kinase activities were assayed in buffers containing substrate, enzyme, and inhibitor at 30 °C in the presence of 15 uM ATP/ [gamma-32P] ATP. 32...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase 1/G2/mitotic-specific cyclin-B(Spiny starfish)
Lawrence Berkeley National Laboratory
Lawrence Berkeley National Laboratory
Affinity DataIC50: 790nMpH: 7.0 T: 2°CAssay Description:Kinase activities were assayed in buffers containing substrate, enzyme, and inhibitor at 30 °C in the presence of 15 uM ATP/ [gamma-32P] ATP. 32...More data for this Ligand-Target Pair
Affinity DataIC50: 850nMAssay Description:Inhibition of Homo sapiens (human) cyclin-dependent kinase 6More data for this Ligand-Target Pair
Affinity DataIC50: 850nMpH: 7.0 T: 2°CAssay Description:The 96-well flat-bottomed plates were coated with recombinant GST-BAD. After the plates were blocked, the reaction buffer containing test compound an...More data for this Ligand-Target Pair
Affinity DataIC50: 850nMpH: 7.0 T: 2°CAssay Description:Kinase activities were assayed in buffers containing substrate, enzyme, and inhibitor at 30 °C in the presence of 15 uM ATP/ [gamma-32P] ATP. 32...More data for this Ligand-Target Pair
Affinity DataIC50: 851nMAssay Description:Inhibition of PIM1 kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 891nMAssay Description:Inhibition of RSK3 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 900nMAssay Description:Inhibition of recombinant PKM2 (unknown origin) Gly128, Asp177, Asp178, Ser362, Hie78, Be51, Asn75 residuesMore data for this Ligand-Target Pair
Affinity DataIC50: 900nMAssay Description:Inhibition of PKM2 (unknown origin)More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX12(Human)
Universidad De Santiago De Chile
Curated by ChEMBL
Universidad De Santiago De Chile
Curated by ChEMBL
TargetEnoyl-acyl-carrier protein reductase(malaria parasite P. falciparum)
Csir-Central Drug Research Institute
Curated by ChEMBL
Csir-Central Drug Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of Plasmodium falciparum FabIMore data for this Ligand-Target Pair
TargetEnoyl-acyl-carrier protein reductase(malaria parasite P. falciparum)
Csir-Central Drug Research Institute
Curated by ChEMBL
Csir-Central Drug Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of FabIMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15(Human)
Universidad De Santiago De Chile
Curated by ChEMBL
Universidad De Santiago De Chile
Curated by ChEMBL
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of 15-hLO1More data for this Ligand-Target Pair
Target3-hydroxyacyl-[acyl-carrier-protein] dehydratase(malaria parasite P. falciparum)
University of Zurich
Curated by ChEMBL
University of Zurich
Curated by ChEMBL
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of FabZMore data for this Ligand-Target Pair
Affinity DataIC50: 2.60E+3nMT: 2°CAssay Description:The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n...More data for this Ligand-Target Pair
Affinity DataIC50: 2.63E+3nMAssay Description:The kinase assay was performed using the EMD Millipore KinaseProfiler service assay protocol. Aurora B kinase was supplied by EMD Millipore Corp. The...More data for this Ligand-Target Pair
Affinity DataIC50: 2.70E+3nMT: 2°CAssay Description:The p38alpha reaction was carried out by using kinase (12ng per well), ATP (100uM) and incubated for 60 min at 37 C. For the JNK3 assay, kinase (10n...More data for this Ligand-Target Pair
Affinity DataIC50: 2.78E+3nMAssay Description:Inhibition of RSK2 (unknown origin)More data for this Ligand-Target Pair
Target1-deoxy-D-xylulose 5-phosphate reductoisomerase(Escherichia coli (strain K12))
University of Strasburg
University of Strasburg
Affinity DataIC50: 3.50E+3nMAssay Description:H-DXR was pre-incubated during 2 min in the presence of the inhibitors at different concentrations and DXP (480 µM). NADPH (160 µM final concentratio...More data for this Ligand-Target Pair
Affinity DataIC50: 3.79E+3nMAssay Description:Inhibition of RSK1 (unknown origin)More data for this Ligand-Target Pair
Target3-oxoacyl-acyl-carrier protein reductase(malaria parasite P. falciparum)
University of Zurich
Curated by ChEMBL
University of Zurich
Curated by ChEMBL
Affinity DataIC50: 4.10E+3nMAssay Description:Inhibition of FabGMore data for this Ligand-Target Pair
Affinity DataIC50: 4.33E+3nMAssay Description:Inhibition of xanthine oxidase assessed as decrease in uric acid production by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of CDK2More data for this Ligand-Target Pair
TargetSolute carrier organic anion transporter family member 2B1(Human)
Soochow University
Curated by ChEMBL
Soochow University
Curated by ChEMBL
Affinity DataIC50: 6.70E+3nMAssay Description:Inhibition of human OATP2B1 expressed in Flp-In-CHO cells assessed as inhibition of OATP2B1 mediated DBF uptake using DBF as fluorescent substrate in...More data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus type 1)
National Cancer Institute-Bethesda
Curated by ChEMBL
National Cancer Institute-Bethesda
Curated by ChEMBL
Affinity DataIC50: 8.50E+3nMAssay Description:IC50 was measured as concentration required to inhibit 50% of HIV-integrase integrationMore data for this Ligand-Target Pair
Affinity DataIC50: 1.25E+4nMAssay Description:Inhibition of amyloid beta (1 to 42) (unknown origin) fibril formation incubated for 1 hr by thioflavin-T fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+4nMAssay Description:Inhibition of baker's yeast alpha-glucosidase using p-nitrophenyl-alpha-D-glucopyranoside as substrate by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataKi: 1.32E+4nMAssay Description:Inhibition constant of compound against binding of Yeast Glyoxalase IMore data for this Ligand-Target Pair
Affinity DataIC50: 1.39E+4nMAssay Description:Inhibition of p56 lckMore data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+4nMAssay Description:Inhibition of SYKMore data for this Ligand-Target Pair
Affinity DataEC50: 1.90E+4nMAssay Description:Inhibition of SYK in human mast cells assessed as reduction in mast cell degranulationMore data for this Ligand-Target Pair
Affinity DataIC50: 1.96E+4nMpH: 6.0 T: 2°CAssay Description:The assay was carried out at room temperature for 10 min with salivary alpha-amylase, starch, and test compounds. The reducing sugar was determined b...More data for this Ligand-Target Pair
Affinity DataIC50: 2.49E+4nMpH: 7.4 T: 2°CAssay Description:The inhibitory activity of flavonoids toward human DPP III was assayed in a 50 mM Tris-HCl buffer, pH 7.4. In brief, recombinant human DPP III (0.29 ...More data for this Ligand-Target Pair
TargetIntegrase(Human immunodeficiency virus type 1)
National Cancer Institute-Bethesda
Curated by ChEMBL
National Cancer Institute-Bethesda
Curated by ChEMBL
Affinity DataIC50: 2.84E+4nMAssay Description:IC50 was measured as concentration required to inhibit 50% of HIV-integrase cleavageMore data for this Ligand-Target Pair
Affinity DataIC50: 2.86E+4nMAssay Description:Inhibition of human DNA topoisomerase 2 catalytic domain-mediated knotted bacteriophage P4Virl dell0 DNA unknotting by agarose gel electrophoresisMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of Trypanosoma cruzi cruzain by Flexstation microplate spectrofluorimetryMore data for this Ligand-Target Pair
Affinity DataIC50: 3.24E+4nMpH: 7.45 T: 2°CAssay Description:Inhibition assay of human testes mcrosomal 17 beta-hydroxysteroid dehydrogenase for the reduction of androstenedione.More data for this Ligand-Target Pair
Affinity DataIC50: 6.00E+4nMAssay Description:Inhibition of Trypanosoma cruzi cruzain by quantitative high throughput screeningMore data for this Ligand-Target Pair
Affinity DataKi: 9.00E+4nMAssay Description:Inhibition of human plasma BChE by Ellman's methodMore data for this Ligand-Target Pair
TargetEndoribonuclease toxin MazF(Escherichia coli (strain K12))
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataEC50: >9.90E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
TargetEndoribonuclease toxin MazF(Escherichia coli (strain K12))
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataEC50: >9.90E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15B(Human)
Universidad De Santiago De Chile
Curated by ChEMBL
Universidad De Santiago De Chile
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of 15-hLO2More data for this Ligand-Target Pair
Affinity DataKi: 1.00E+5nMAssay Description:Inhibition of human recombinant AChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.89E+5nMAssay Description:In vitro antibacterial activity was determined as inhibitory concentration causing 50% DNA-gyrase supercoiling inhibition (SCI)More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)