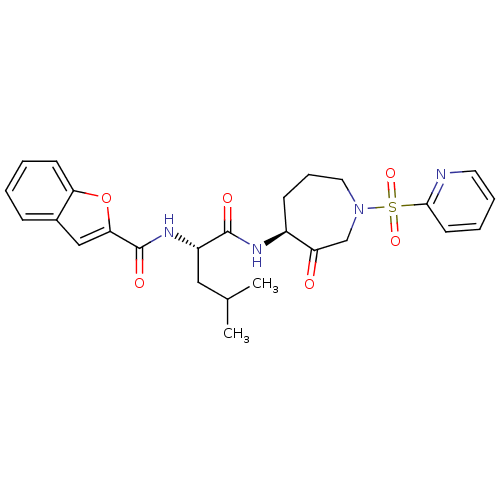
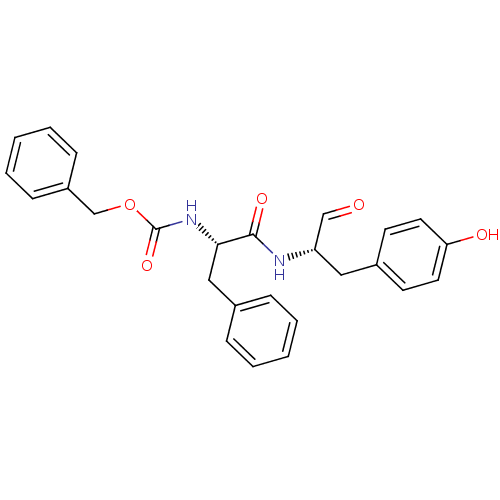
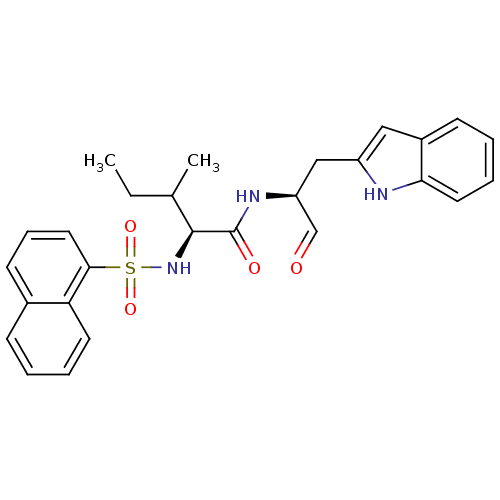
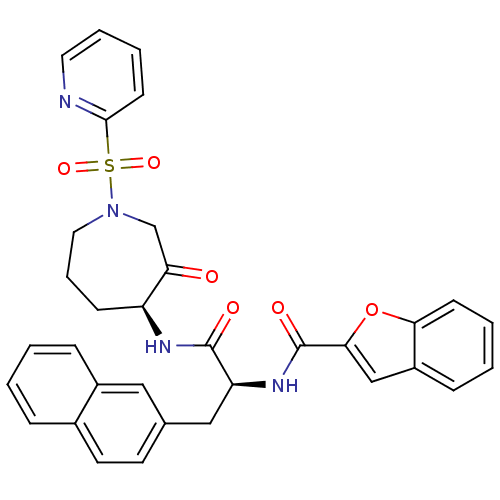
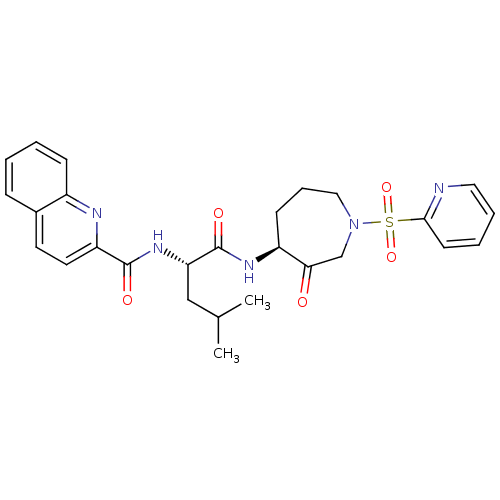
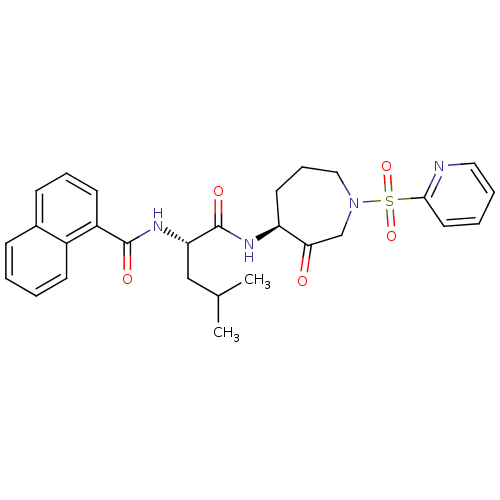
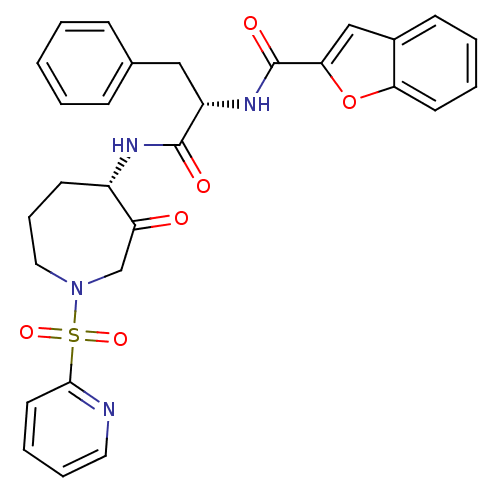
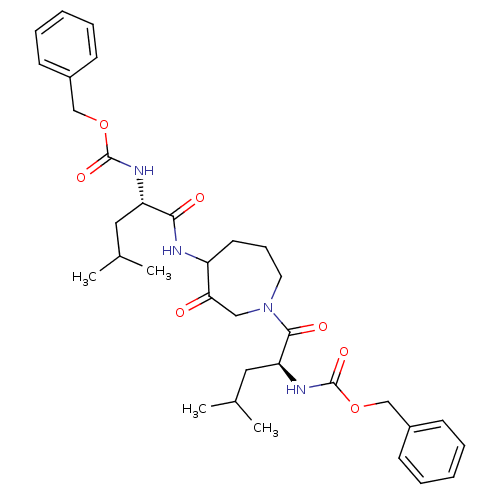
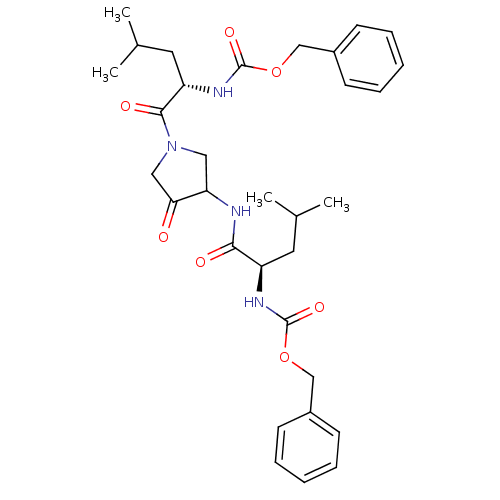
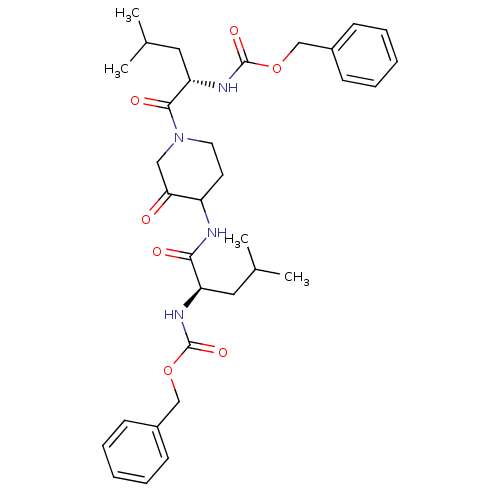
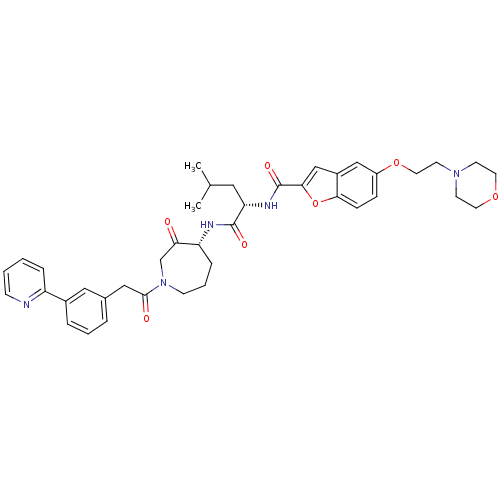
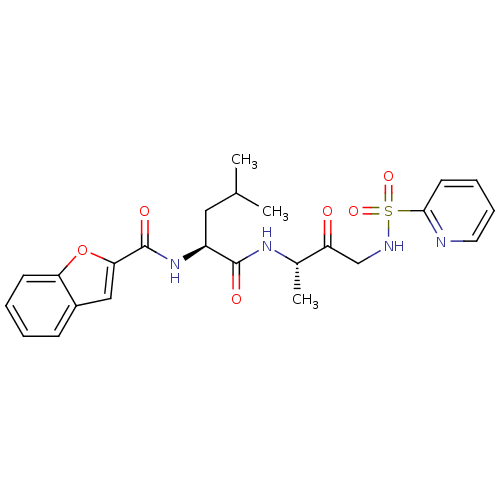
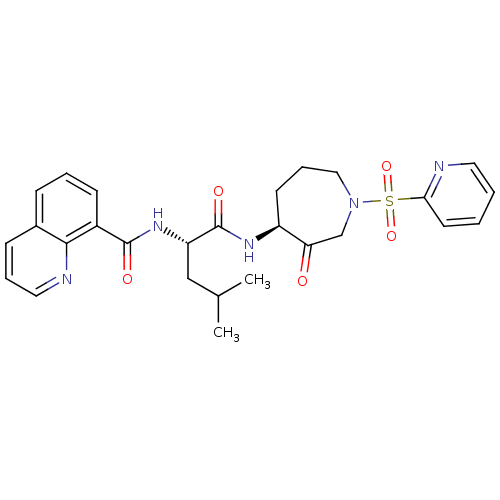
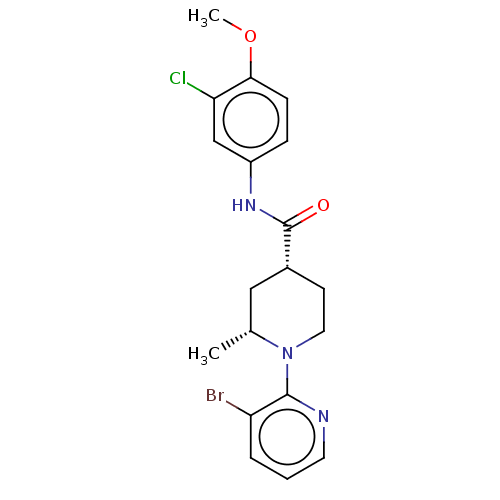
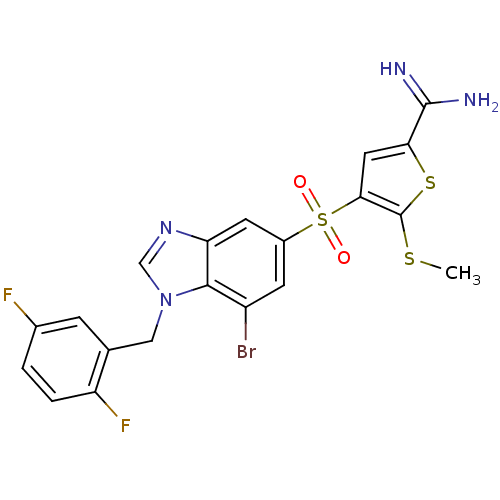
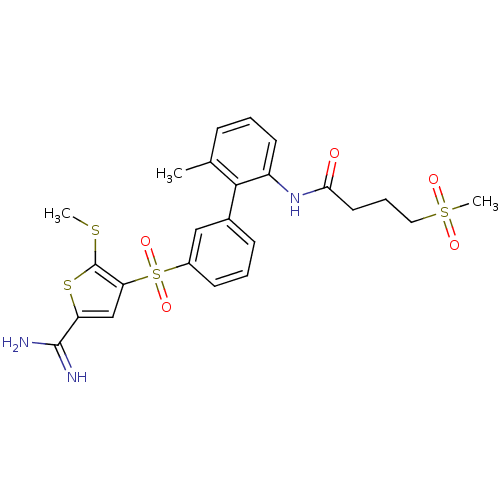
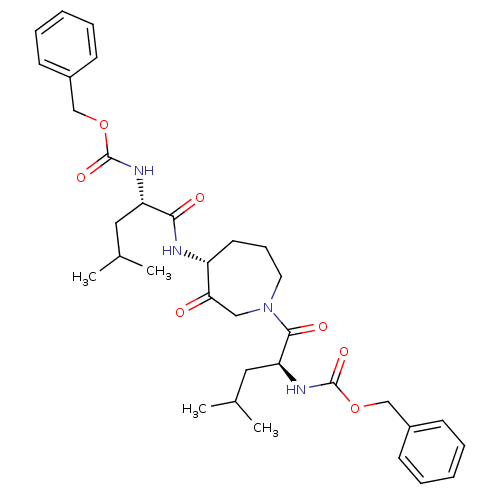
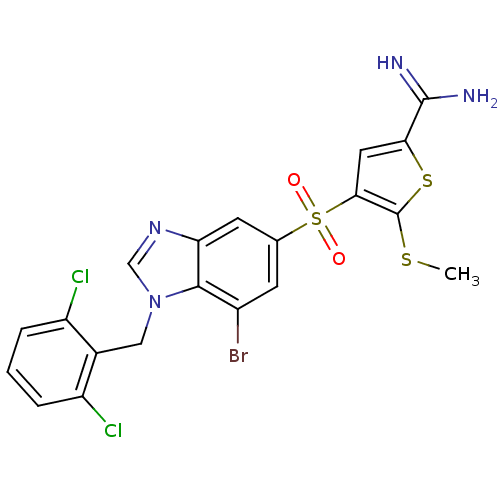
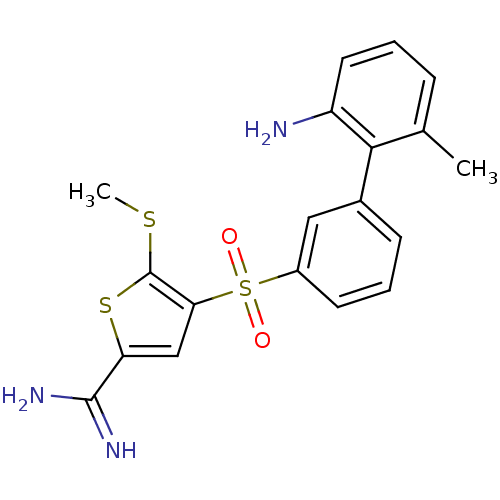
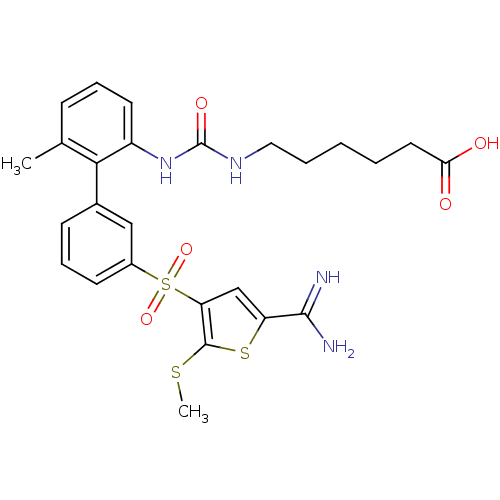
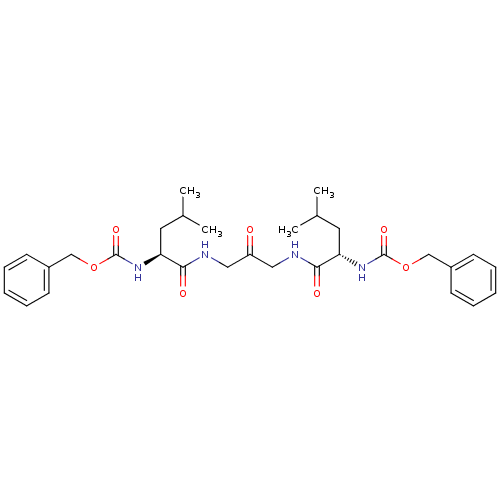
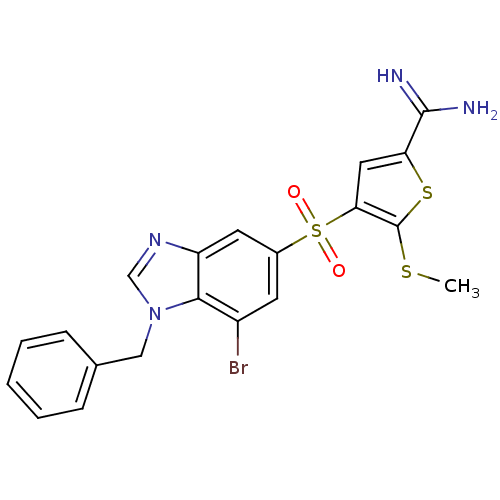
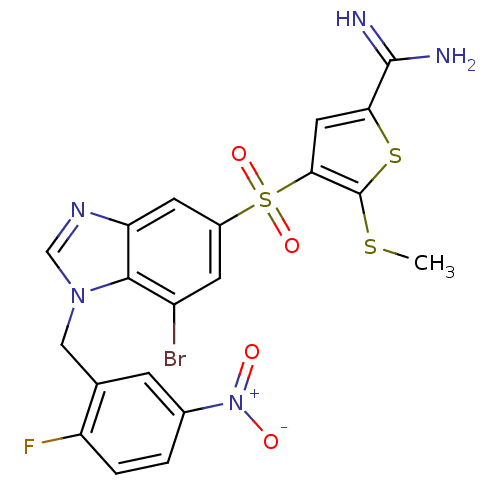
Affinity DataKi: 0.00480nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 0.160nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 0.160nMAssay Description:Apparent inhibitory constant against human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 0.260nMAssay Description:Apparent inhibitory constant against human cathepsin SMore data for this Ligand-Target Pair
Affinity DataKi: 0.430nMAssay Description:Apparent inhibitory constant against human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 0.470nMAssay Description:Apparent inhibitory constant against human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 0.490nMAssay Description:Inhibitory activity of the compound against Human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 0.560nMAssay Description:Apparent inhibitory constant against human cathepsin SMore data for this Ligand-Target Pair
Affinity DataKi: 0.570nMAssay Description:Apparent inhibitory constant against human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 0.740nMAssay Description:Apparent inhibitory constant against human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 0.870nMAssay Description:Apparent inhibitory constant against human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 0.890nMAssay Description:Apparent inhibitory constant against human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 1.40nMAssay Description:Apparent inhibitory constant against human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 1.70nMAssay Description:Apparent inhibitory constant against human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Apparent inhibitory constant against human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 2.20nMAssay Description:Apparent inhibitory constant against human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 2.20nMAssay Description:Inhibitory activity of the compound against Human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 2.30nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 2.60nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 3.10nMAssay Description:Apparent inhibitory constant against human cathepsin SMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Inhibitory activity of the compound against Human cathepsin SMore data for this Ligand-Target Pair
Affinity DataKi: 4.30nMAssay Description:Apparent inhibitory constant against human cathepsin SMore data for this Ligand-Target Pair
Affinity DataKi: 4.70nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 4.80nMAssay Description:Inhibitory activity of the compound against Rat cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 5nMAssay Description:Apparent inhibitory constant against human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 5.70nMAssay Description:Apparent inhibitory constant against human cathepsin SMore data for this Ligand-Target Pair
Affinity DataKi: 7.5nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 8.20nMAssay Description:Apparent inhibitory constant against human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 8.90nMAssay Description:Apparent inhibitory constant against human cathepsin SMore data for this Ligand-Target Pair
TargetMucosa-associated lymphoid tissue lymphoma translocation protein 1(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataKi: 9.60nMAssay Description:Non-competitive inhibition of strep-tagged and His-tagged full length MALT1 protease (1 to 824 residues) (unknown origin) expressed in baculovirus in...More data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 11nMAssay Description:Apparent inhibitory constant against human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 12nMAssay Description:Apparent inhibitory constant against human cathepsin KMore data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 14nMAssay Description:Inhibition of Complement C1s subcomponentMore data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Inhibitory activity of the compound against Human cathepsin SMore data for this Ligand-Target Pair
Affinity DataKi: 15nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Inhibitory activity of the compound against Human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Apparent inhibitory constant against human cathepsin SMore data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 20nMAssay Description:Inhibitory activity of the compound against Human cathepsin SMore data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 20nMAssay Description:Inhibition of Complement C1s subcomponentMore data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 22nMAssay Description:Inhibition of Complement C1s subcomponentMore data for this Ligand-Target Pair
Affinity DataKi: 22nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 24nMAssay Description:Apparent inhibitory constant against human cathepsin BMore data for this Ligand-Target Pair
Affinity DataKi: 26nMAssay Description:Inhibitory activity of the compound against Human cathepsin SMore data for this Ligand-Target Pair
Affinity DataKi: 27nMAssay Description:Apparent inhibitory constant against human cathepsin SMore data for this Ligand-Target Pair
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
TargetComplement C1s subcomponent(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL

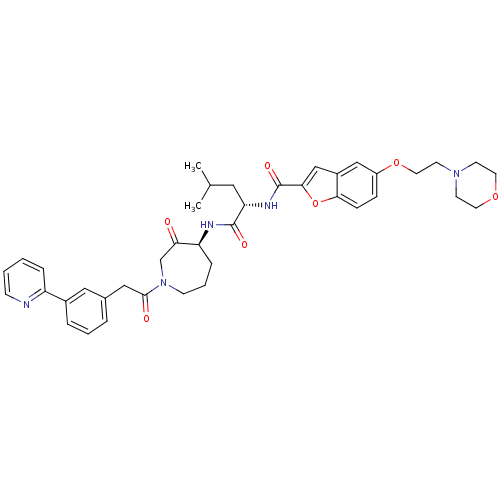
 3D Structure (crystal)
3D Structure (crystal)