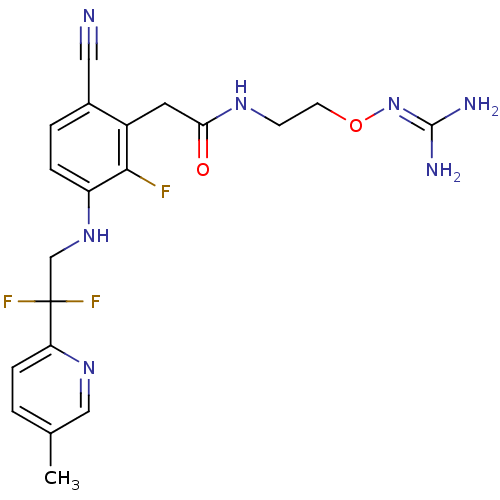
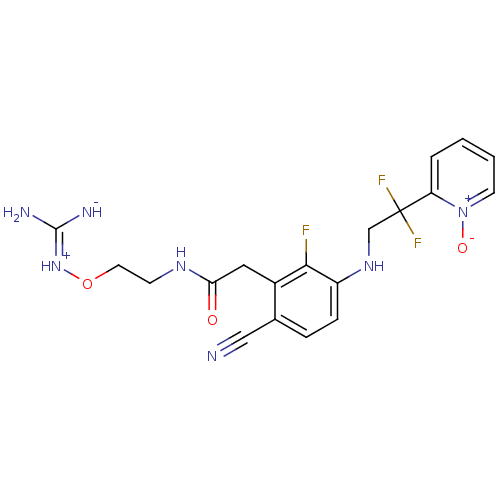
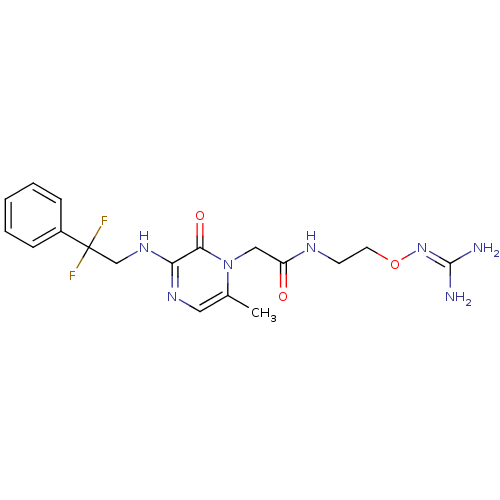
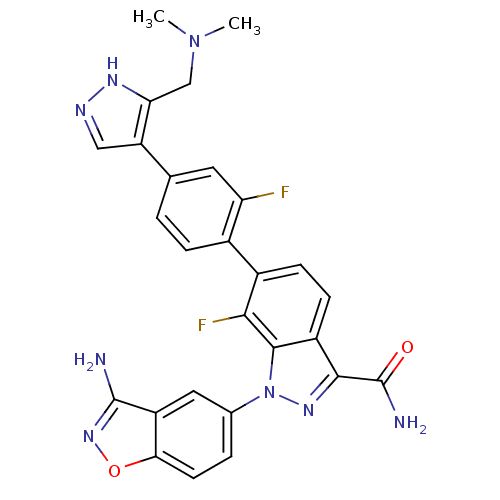
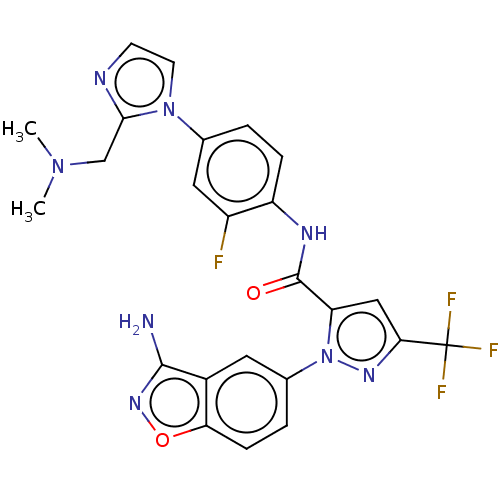
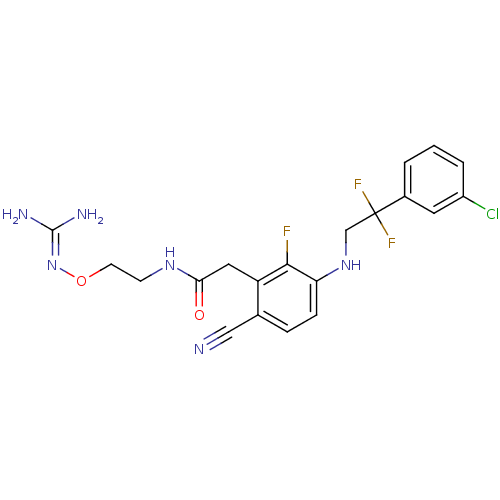
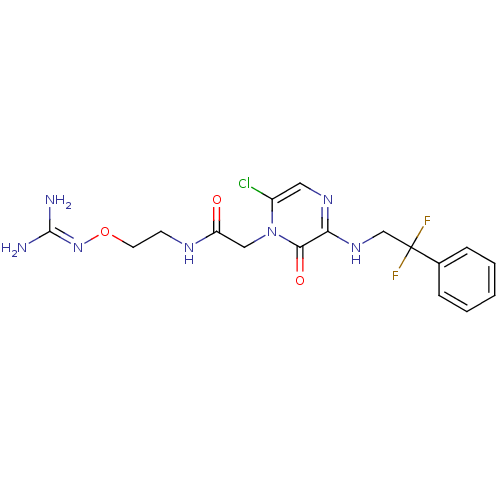
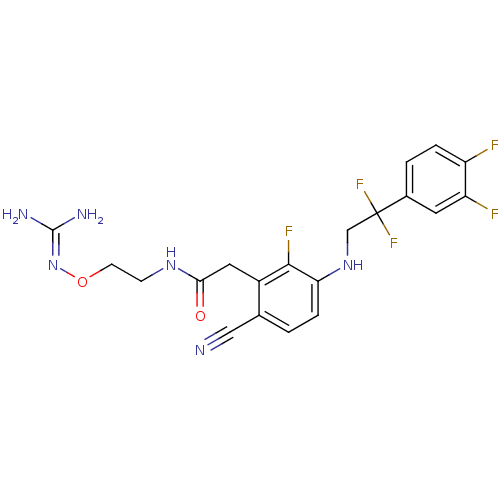
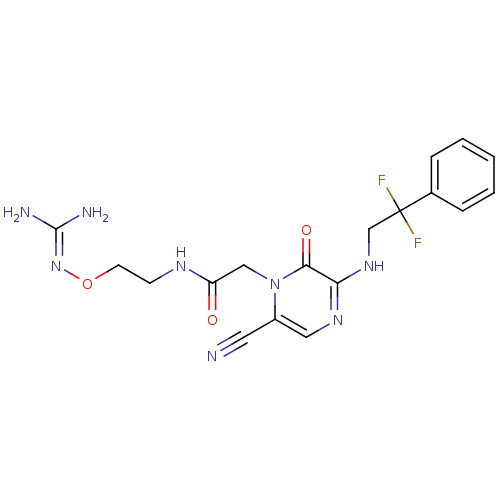
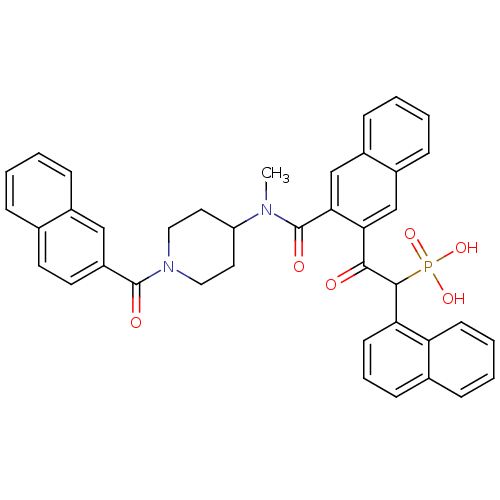
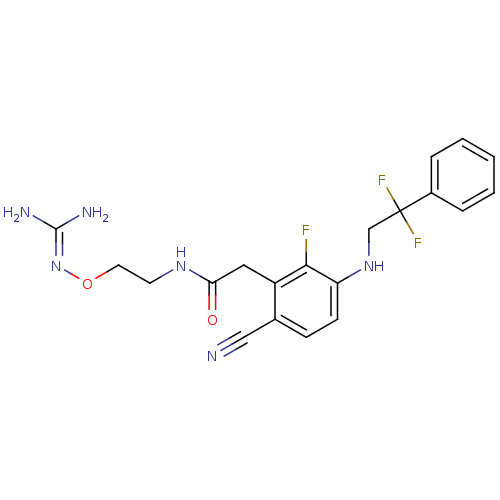
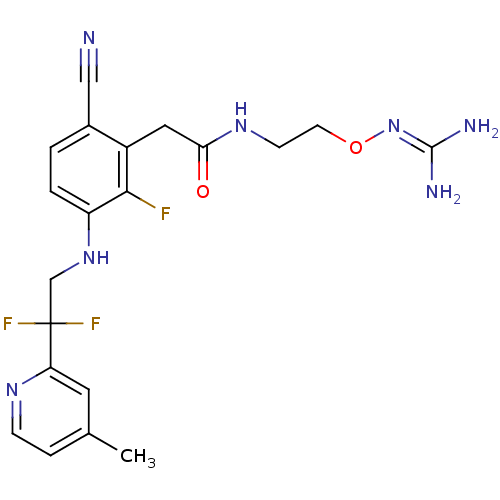
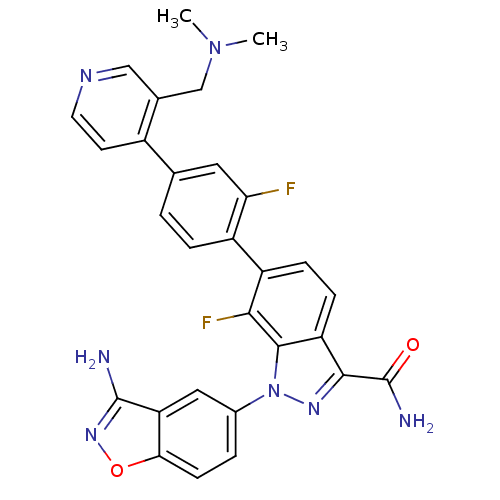
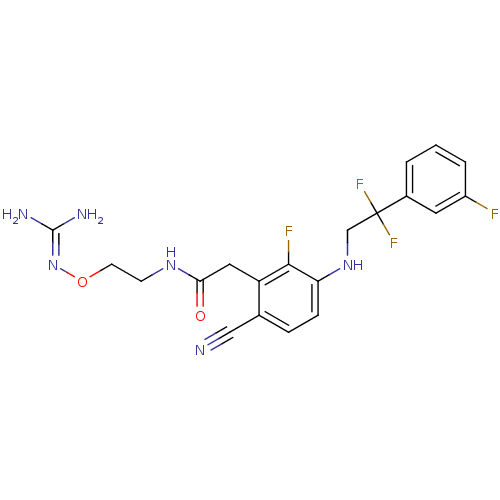
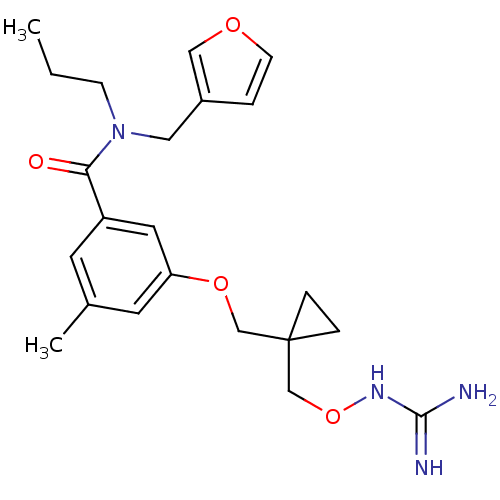
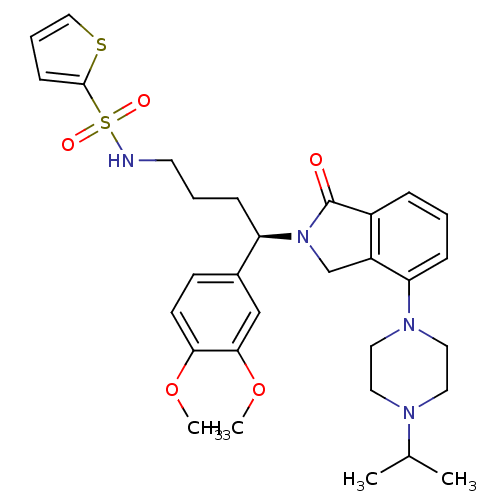
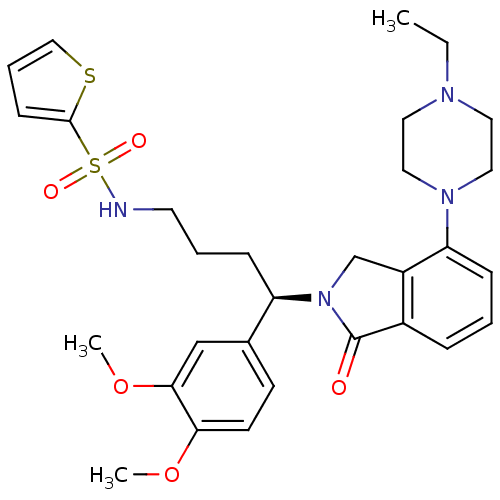
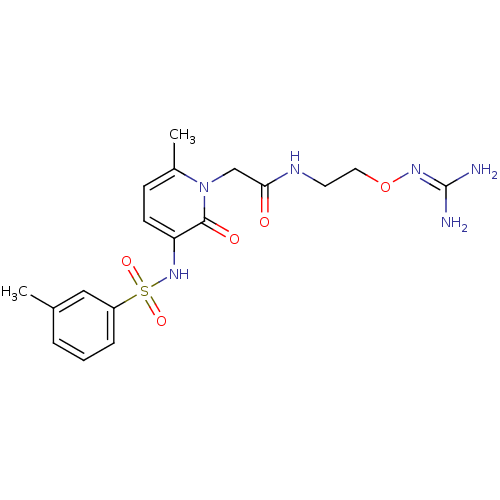
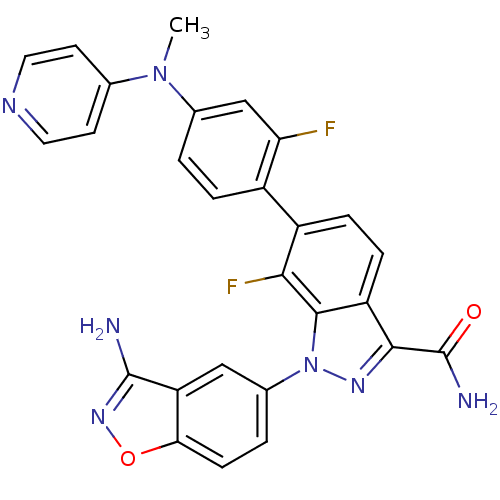
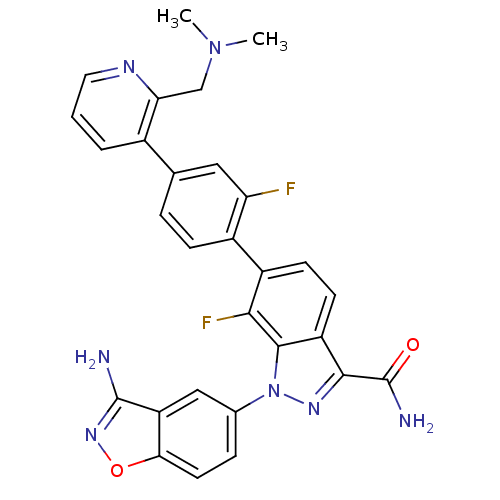
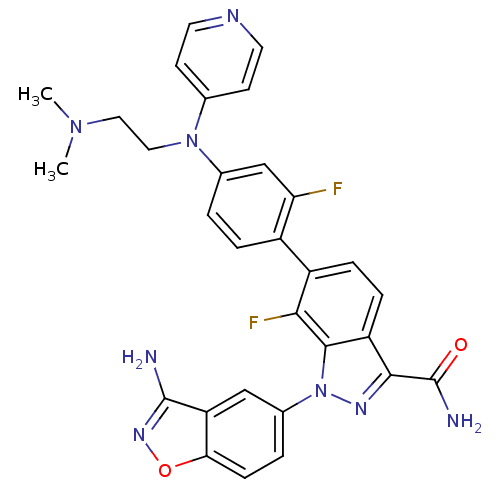
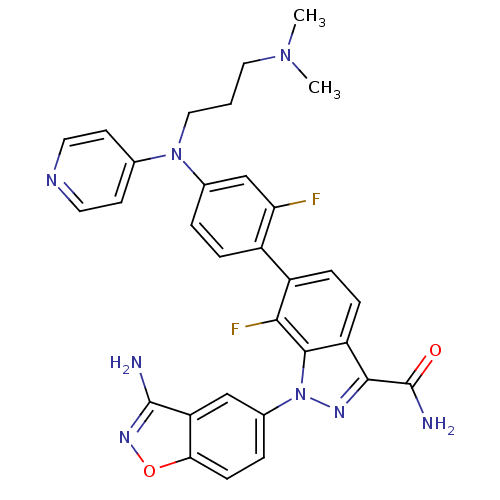
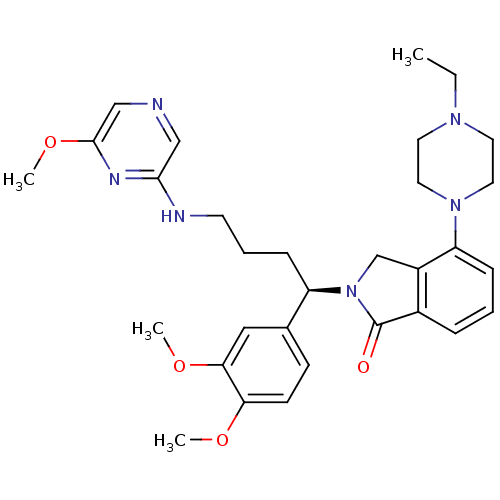
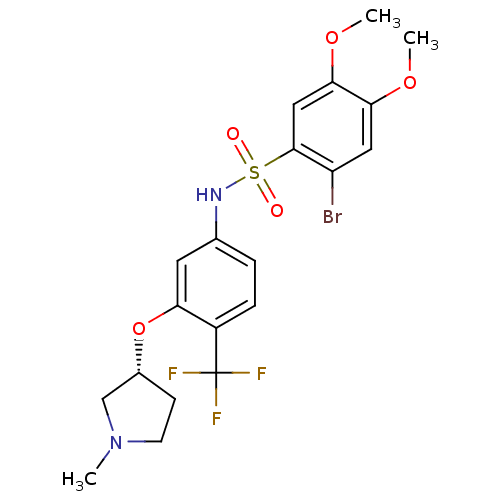
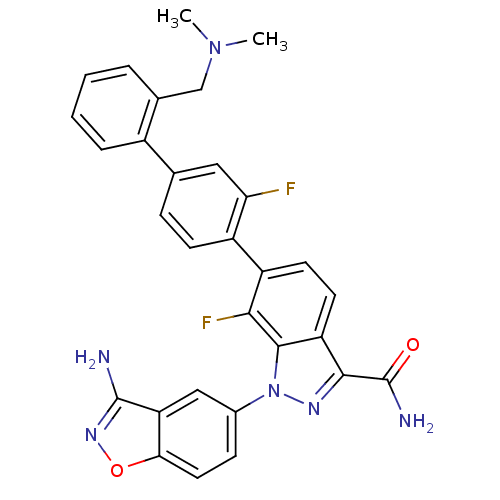
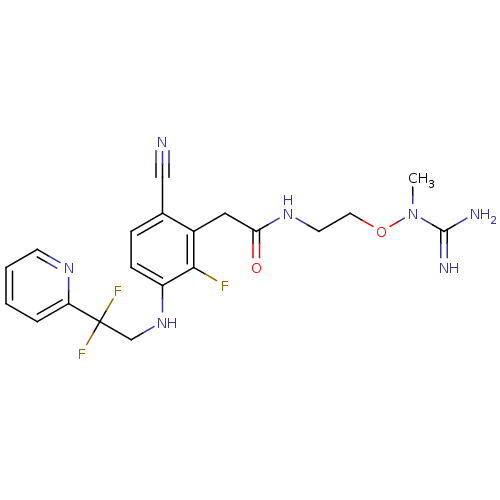
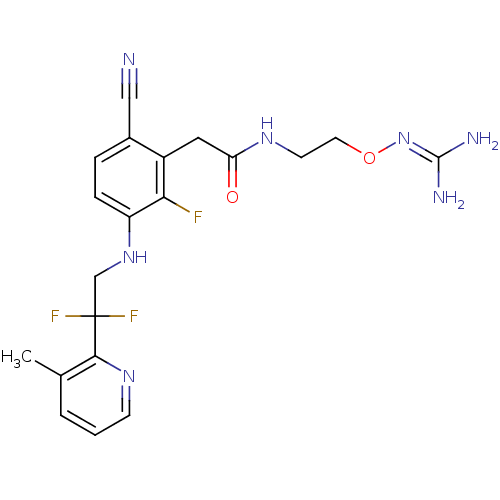
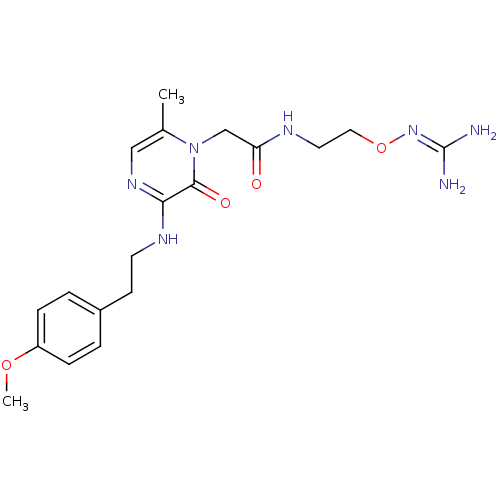
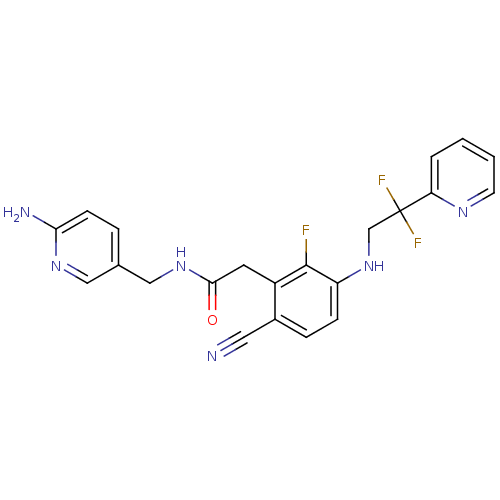
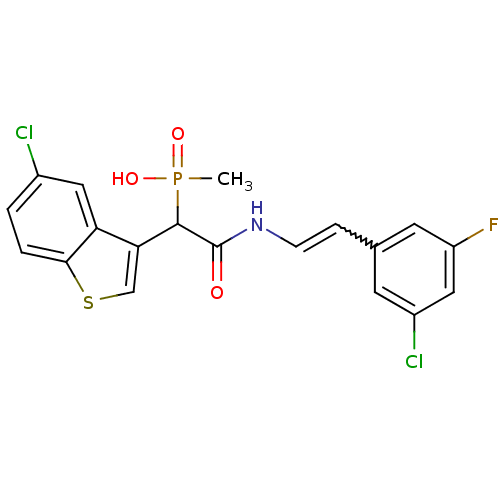
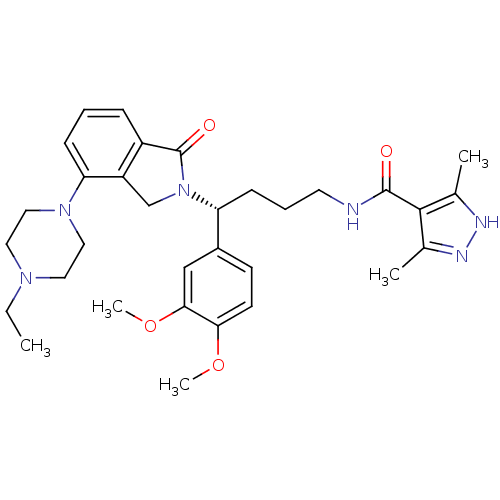
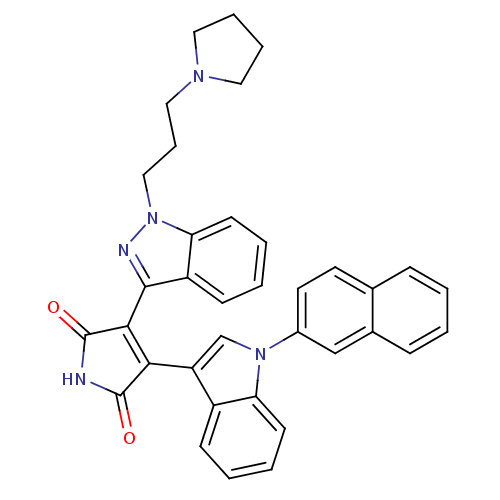
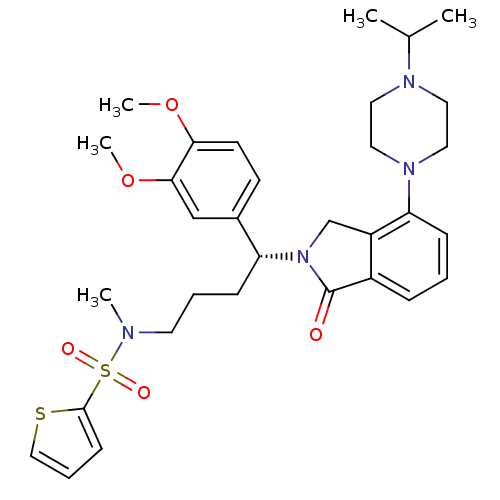
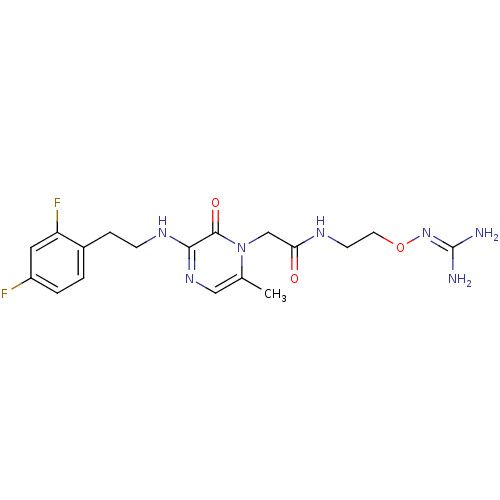
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
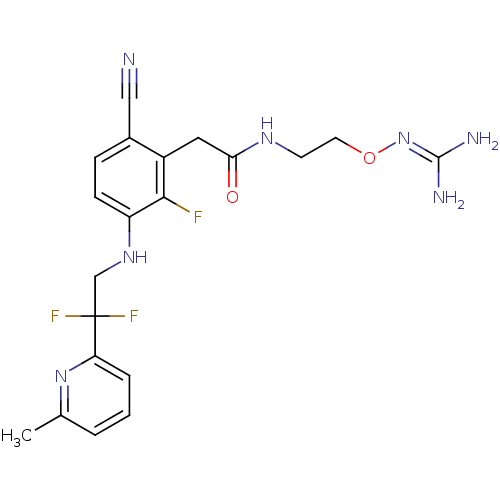
Affinity DataKi: 0.100nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
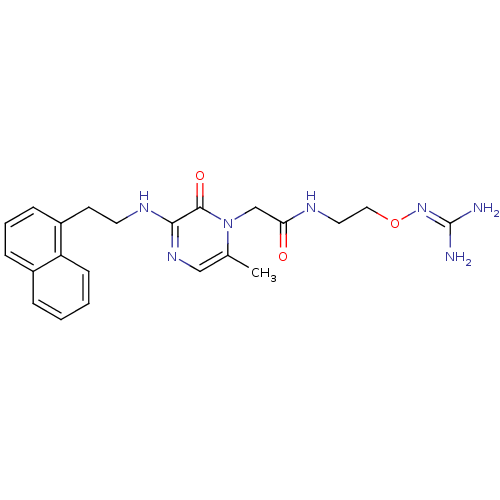
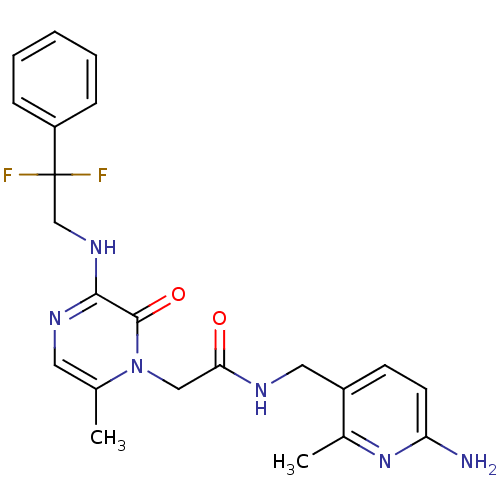
TargetUrotensin-2 receptor(RAT)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.120nMAssay Description:Displacement of [125I]urotensin 2 from rat urotensin 2 receptor expressed in CHOK1 cells by scintillation proximity assayMore data for this Ligand-Target Pair
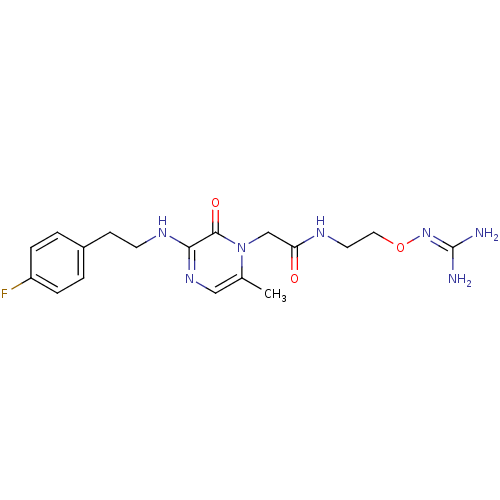
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.380nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
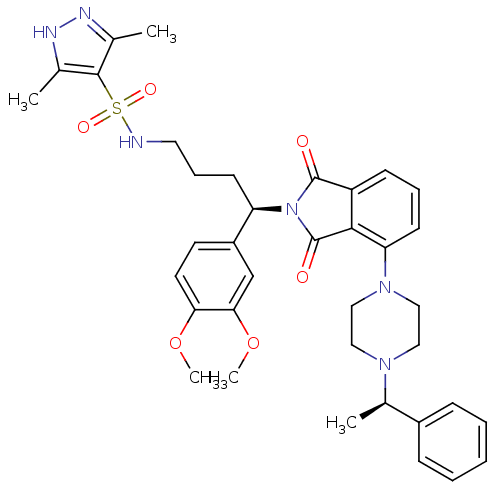
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.570nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.650nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.660nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.770nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 0.790nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.20nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.20nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.30nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.30nMAssay Description:Inhibition of human alpha-thrombinMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1.40nMAssay Description:Inhibition of human factor 10aMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1.60nMAssay Description:Inhibition of human factor 10aMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1.60nMAssay Description:Inhibition of human factor 10aMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.70nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.70nMAssay Description:Inhibition of human alpha-thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 1.80nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2.30nMAssay Description:Inhibition of human alpha-thrombinMore data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 2.30nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2.30nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 2.90nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Inhibition of human factor 10aMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 3.20nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Binding affinity to thrombinMore data for this Ligand-Target Pair
TargetUrotensin-2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Displacement of [125I]urotensin 2 from urotensin 2 receptor in human RMS13 cells by scintillation proximity assayMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetUrotensin-2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Displacement of [125I]urotensin 2 from urotensin 2 receptor in human RMS13 cells by scintillation proximity assayMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 4.10nMAssay Description:Inhibition of human factor 10aMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 4.40nMAssay Description:Inhibition of human factor 10aMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 4.90nMAssay Description:Inhibition of human factor 10aMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 5.20nMAssay Description:Inhibition of human factor 10aMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 6.40nMAssay Description:Inhibition of human factor 10aMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 6.80nMAssay Description:Inhibition of human alpha-thrombinMore data for this Ligand-Target Pair
TargetUrotensin-2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 8nMAssay Description:Displacement of [125I]urotensin 2 from urotensin 2 receptor in human RMS13 cells by scintillation proximity assayMore data for this Ligand-Target Pair
TargetUrotensin-2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 8nMAssay Description:Displacement of [125I]urotensin 2 from urotensin 2 receptor in human RMS13 cells by scintillation proximity assayMore data for this Ligand-Target Pair
TargetCoagulation factor X(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 8.10nMAssay Description:Inhibition of human factor 10aMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 9nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 11nMAssay Description:Inhibition of human alpha-thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 11nMAssay Description:Binding affinity to human thrombinMore data for this Ligand-Target Pair
TargetChymase(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 11nMAssay Description:Inhibition of human skin chymaseMore data for this Ligand-Target Pair
TargetUrotensin-2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 11nMAssay Description:Displacement of [125I]urotensin 2 from urotensin 2 receptor in human RMS13 cells by scintillation proximity assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 11nMAssay Description:Inhibition of Potassium channel HERGMore data for this Ligand-Target Pair
TargetUrotensin-2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 11nMAssay Description:Displacement of [125I]urotensin 2 from urotensin 2 receptor in human RMS13 cells by scintillation proximity assayMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 12nMAssay Description:Inhibition of human alpha-thrombinMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 13nMAssay Description:Inhibition of human alpha-thrombinMore data for this Ligand-Target Pair
TargetUrotensin-2 receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 13nMAssay Description:Displacement of [125I]urotensin 2 from urotensin 2 receptor in human RMS13 cells by scintillation proximity assayMore data for this Ligand-Target Pair
TargetProthrombin(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 15nMAssay Description:Inhibition of human alpha-thrombinMore data for this Ligand-Target Pair

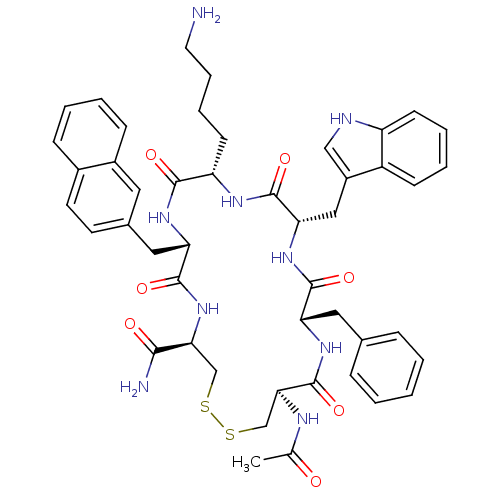
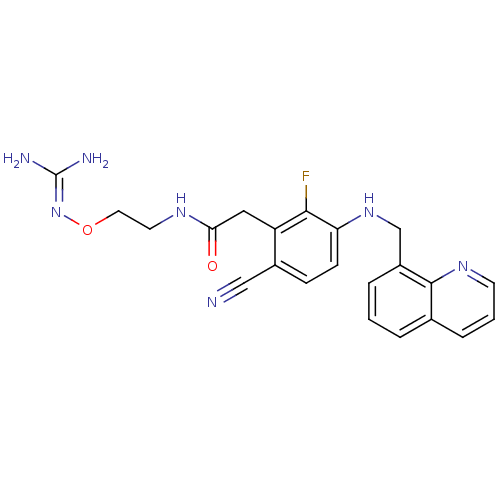
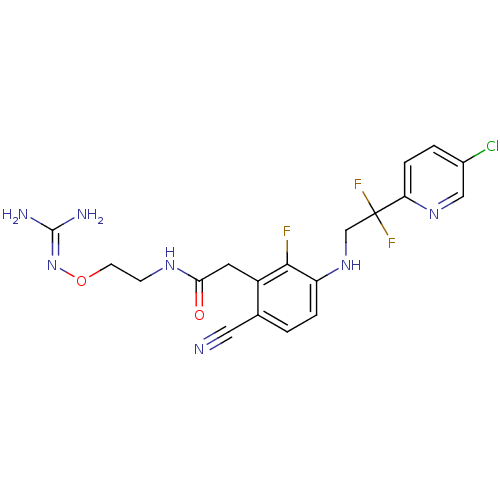
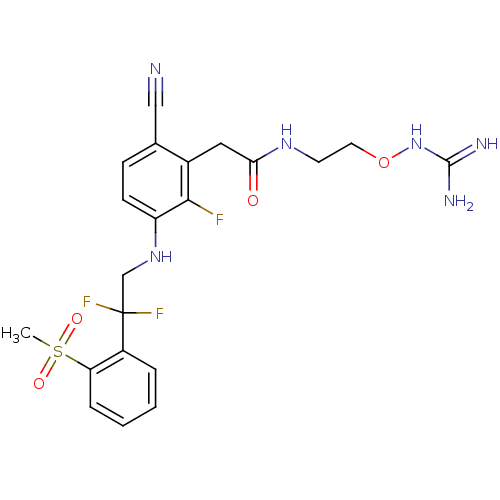
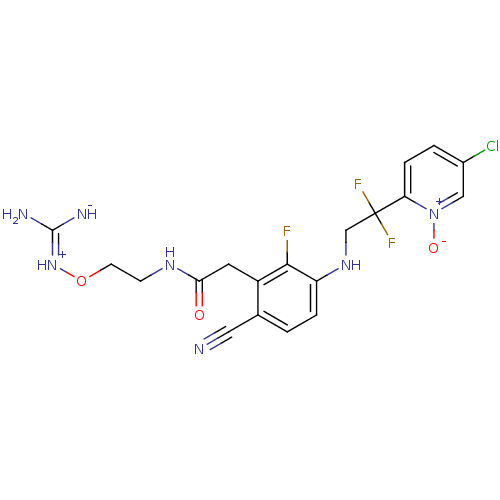
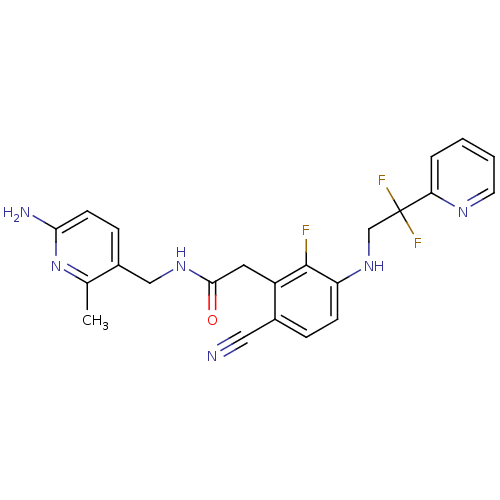

 3D Structure (crystal)
3D Structure (crystal)