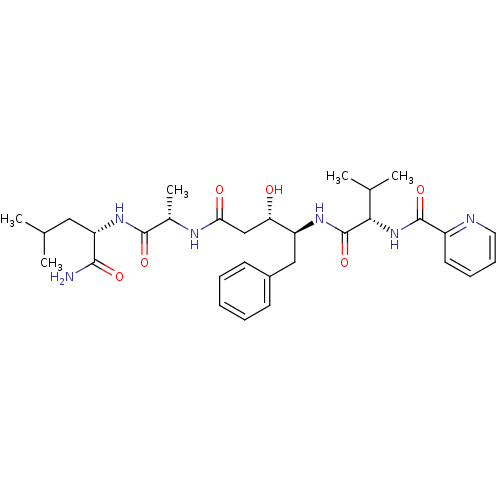
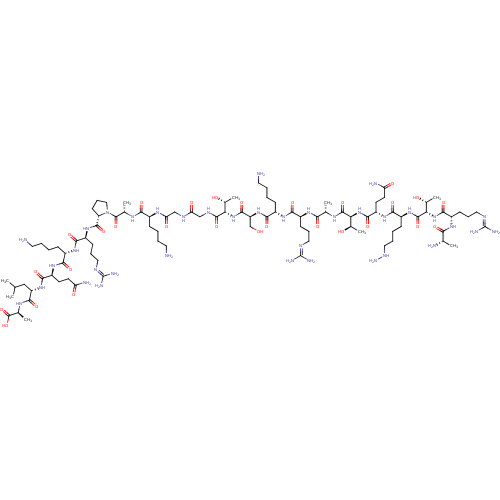
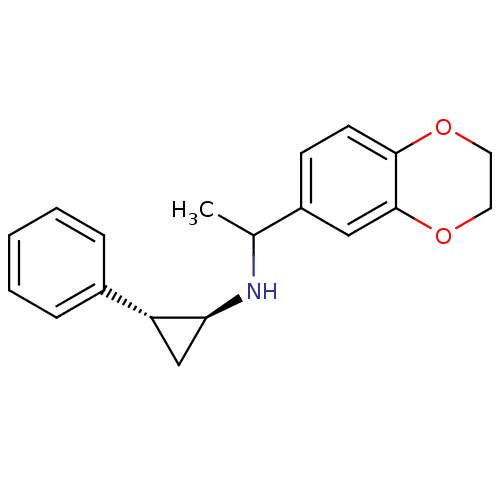
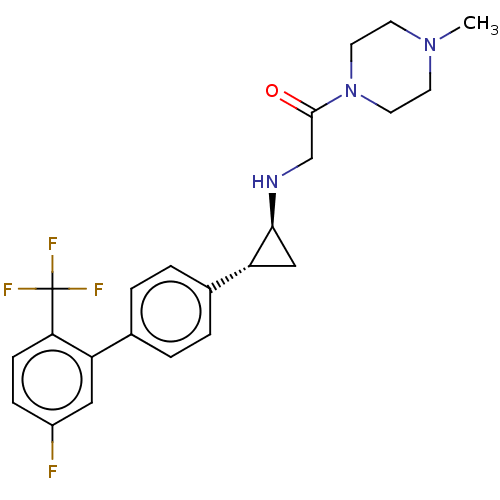
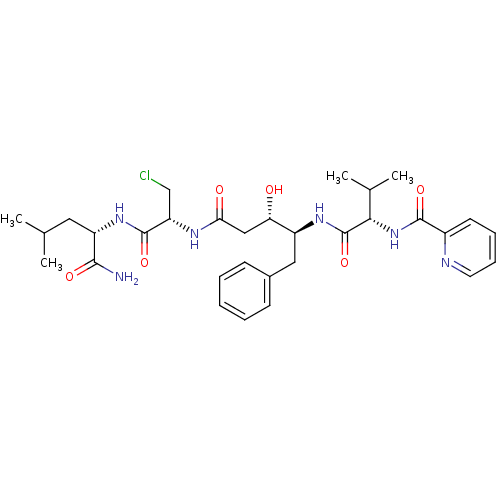
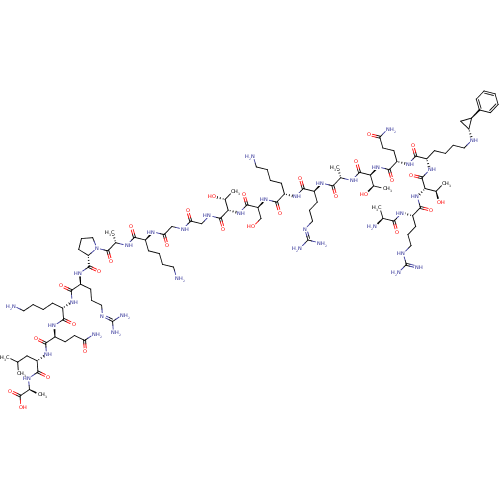
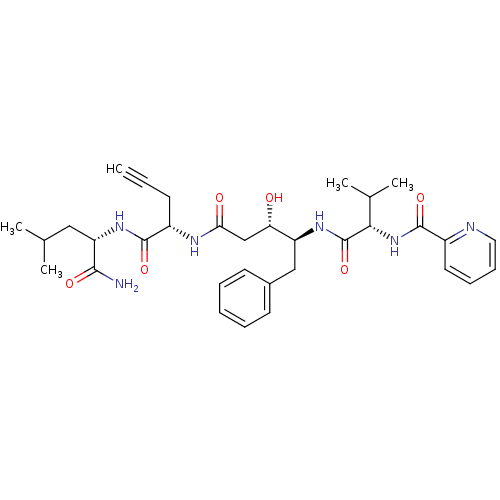
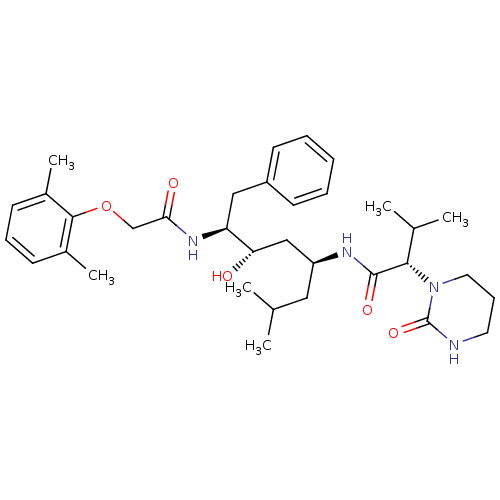
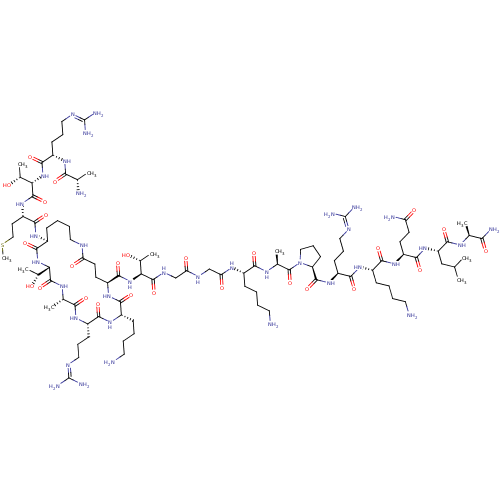
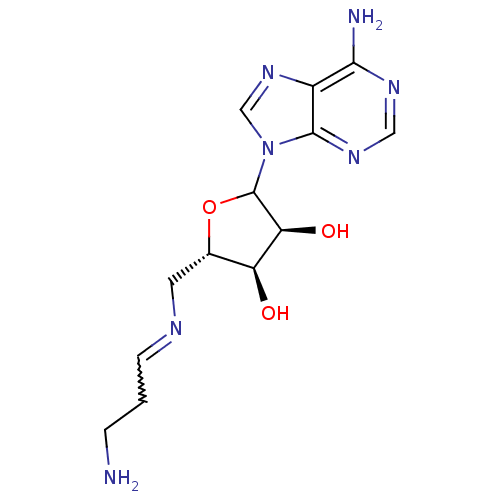
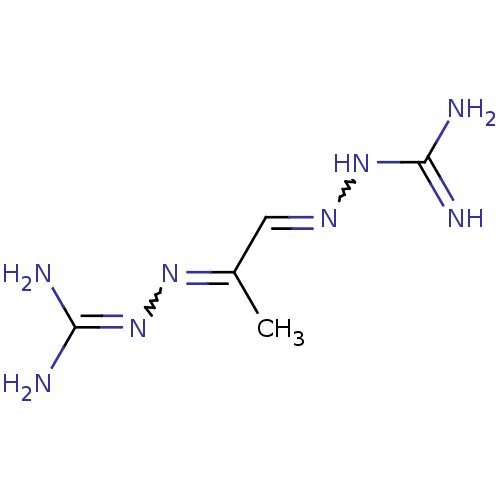
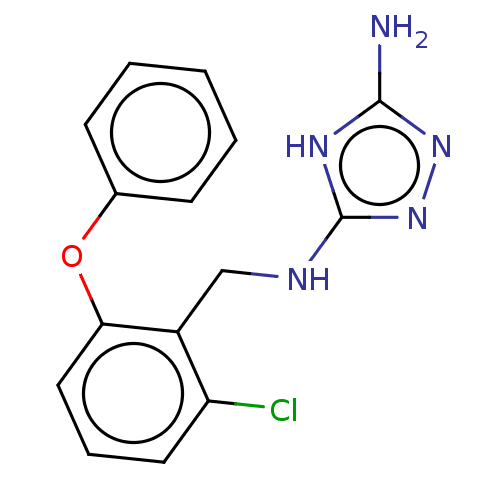
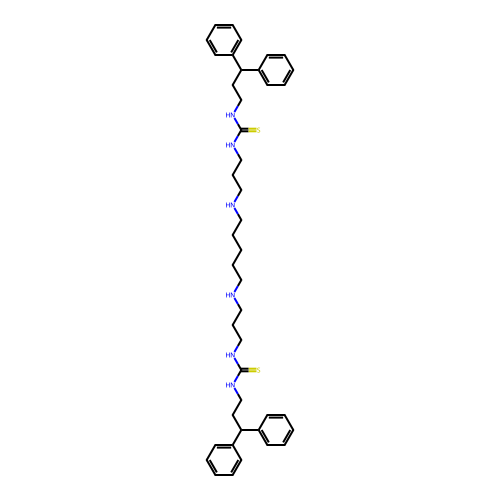
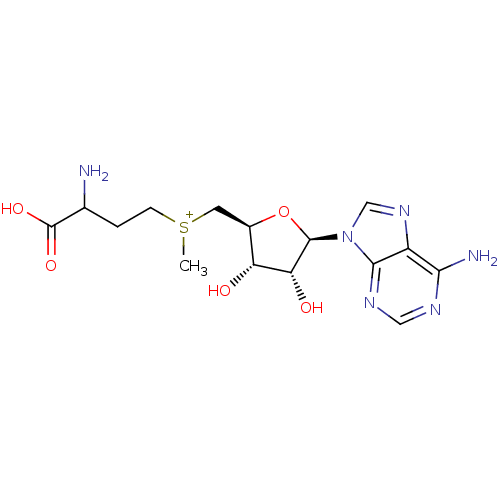
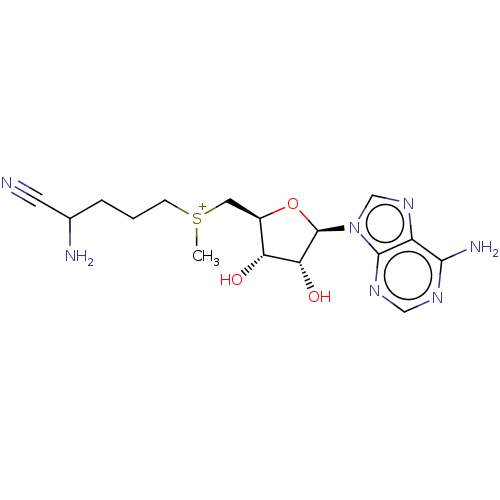
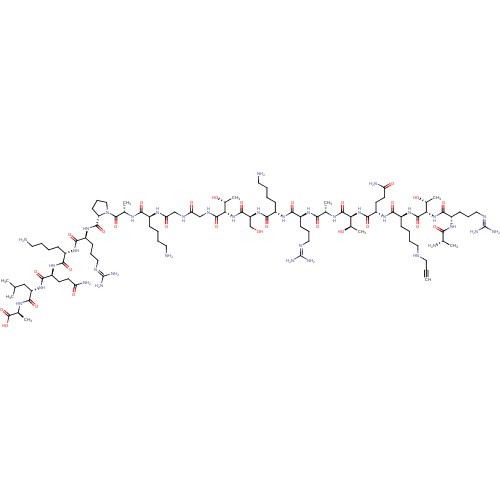
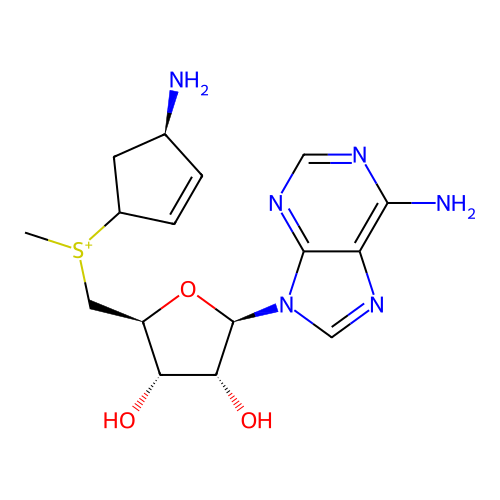
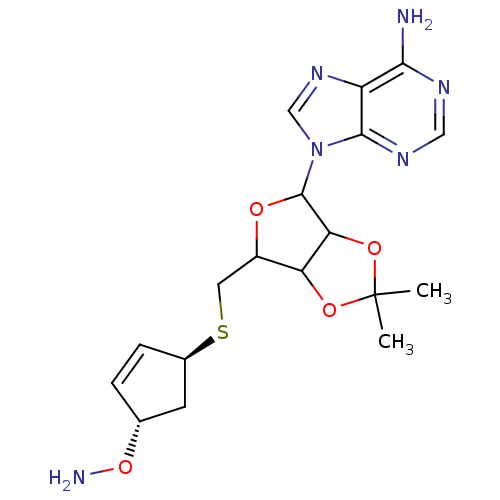
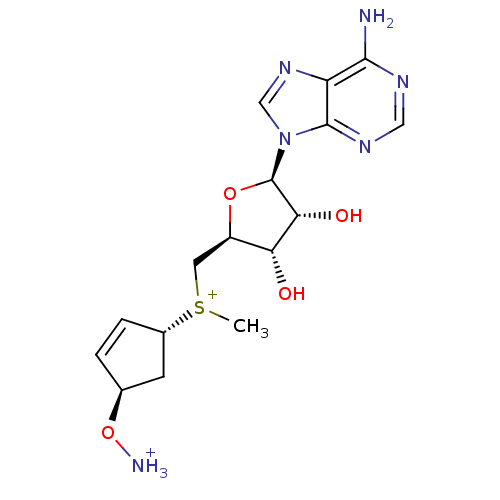
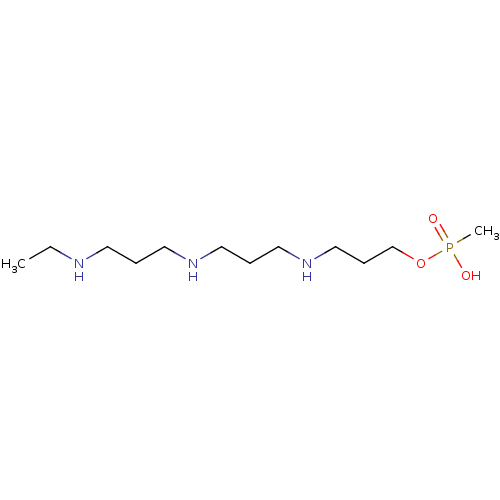
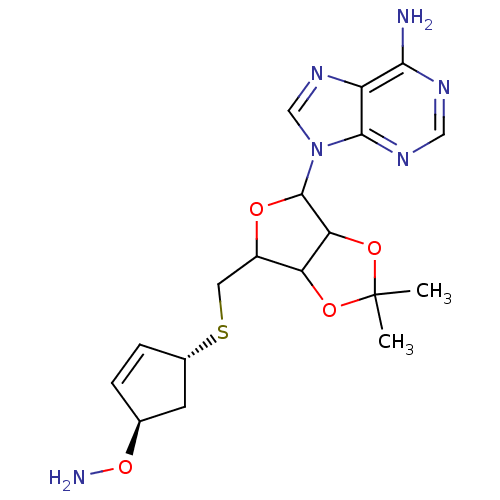
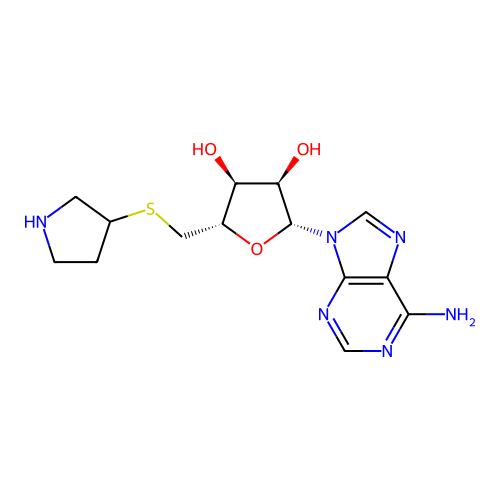
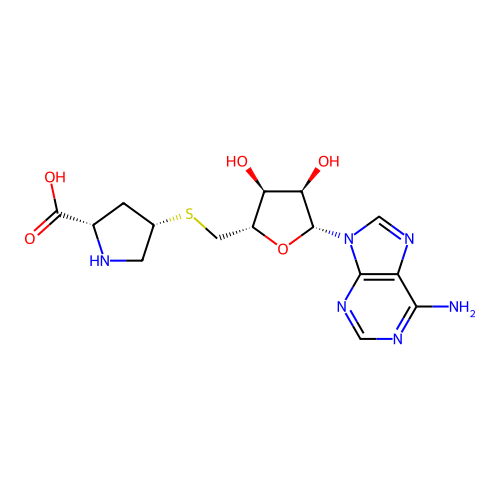
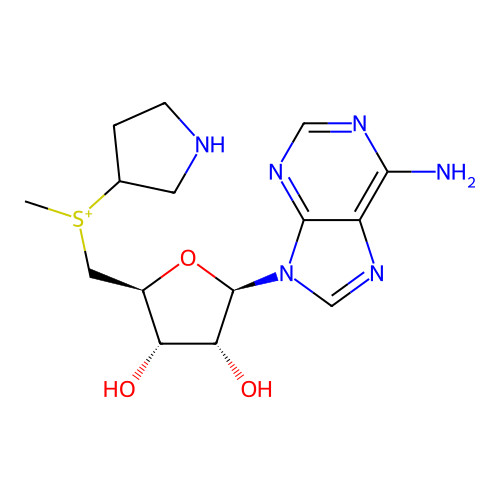
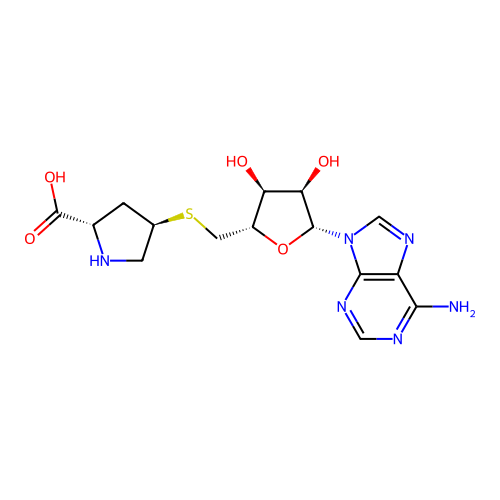
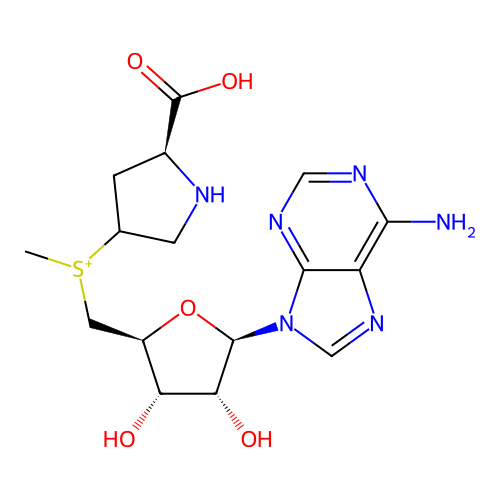
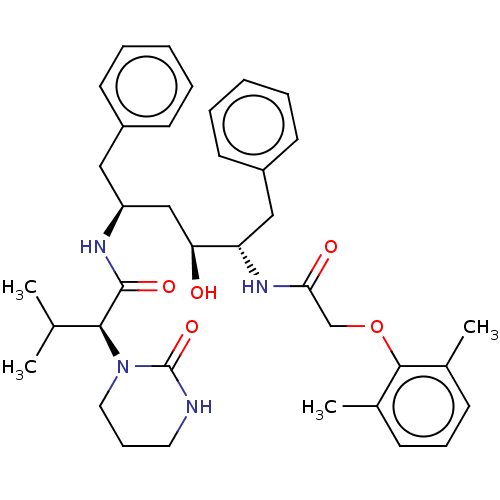
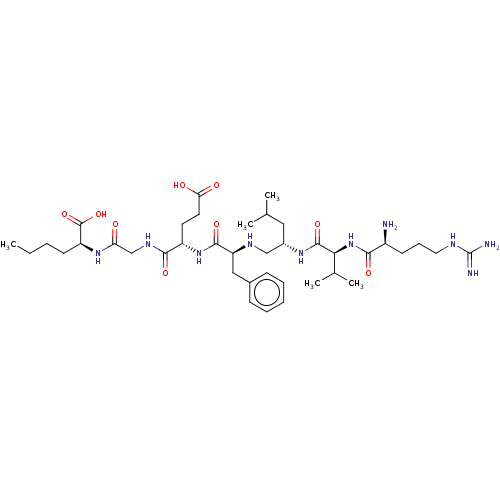
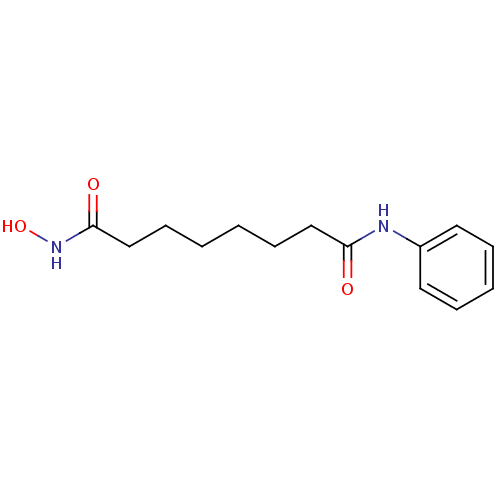
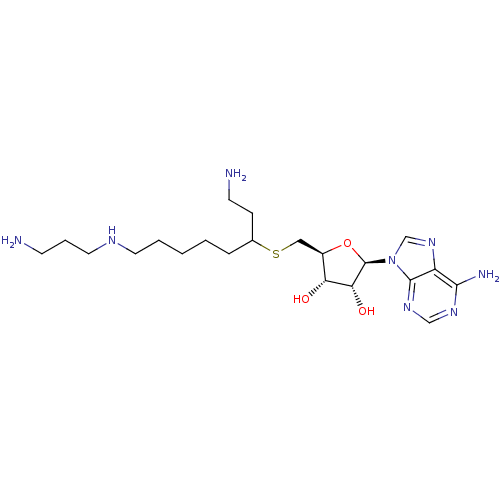
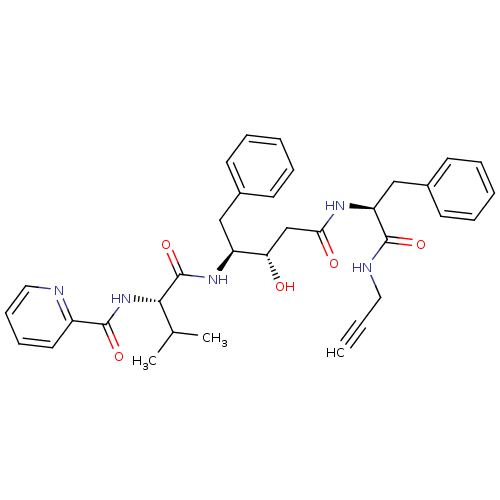
Affinity DataKi: 0.5nMAssay Description:Inhibition of Plasmodium falciparum plasmepsin 2More data for this Ligand-Target Pair
Affinity DataKi: 0.560nMAssay Description:Inhibition of Plasmodium falciparum recombinant plasmepsin 2 expressed in Escherichia coli BL21 (DE3)More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Medical University Of South Carolina
Curated by ChEMBL
Medical University Of South Carolina
Curated by ChEMBL
Affinity DataKi: 4.40nMAssay Description:Inhibition of recombinant LSD1 (178 to 831) (unknown origin) expressed in baculovirus infected insect Sf9 cells using diMeK4H3-21 as substrate by per...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Medical University Of South Carolina
Curated by ChEMBL
Medical University Of South Carolina
Curated by ChEMBL
Affinity DataKi: 5nMAssay Description:Inhibition of human recombinant LSD1 using di-methylated H3-K4 peptide as substrate assessed as release of H2O2 preincubated for 15 mins followed by ...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Medical University Of South Carolina
Curated by ChEMBL
Medical University Of South Carolina
Curated by ChEMBL
Affinity DataKi: 9nMAssay Description:Inhibition of human recombinant LSD1 using di-methylated H3-K4 peptide as substrate assessed as release of H2O2 preincubated for 15 mins followed by ...More data for this Ligand-Target Pair
Affinity DataKi: 35.7nMAssay Description:Inhibition of Plasmodium falciparum plasmepsin 2 expressed in Escherichia coli BL21 (DE3) after 40 mins by Kitz-Wilson plot analysisMore data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Medical University Of South Carolina
Curated by ChEMBL
Medical University Of South Carolina
Curated by ChEMBL
Affinity DataKi: 40nMAssay Description:Inhibition of recombinant human LSD1 expressed in Escherichia coli using methylated H3-K4 peptide as substrate by peroxidase-coupled assayMore data for this Ligand-Target Pair
Affinity DataKi: 40nMAssay Description:Inhibition of LSD1/CoREST (unknown origin)More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A/REST corepressor 1 [4-485](Homo sapiens (Human))
Medical University Of South Carolina
Curated by ChEMBL
Medical University Of South Carolina
Curated by ChEMBL
Affinity DataKi: 50nMAssay Description:Inhibition of recombinant human LSD1/CoREST complex expressed in Escherichia coli using methylated H3-K4 peptide as substrate by peroxidase-coupled a...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Medical University Of South Carolina
Curated by ChEMBL
Medical University Of South Carolina
Curated by ChEMBL
Affinity DataKi: 107nMAssay Description:Inhibition of recombinant LSD1 (178 to 831) (unknown origin) expressed in baculovirus infected insect Sf9 cells using diMeK4H3-21 as substrate by per...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Medical University Of South Carolina
Curated by ChEMBL
Medical University Of South Carolina
Curated by ChEMBL
Affinity DataKi: 120nMAssay Description:Inhibition of recombinant LSD1 (unknown origin) using di-methylated H3-K4 peptide as substrate incubated for 5 mins prior to substrate addition by fl...More data for this Ligand-Target Pair
Affinity DataKi: 121nMAssay Description:Inhibition of Plasmodium falciparum plasmepsin 2More data for this Ligand-Target Pair
Affinity DataKi: 333nMAssay Description:Inhibition of Plasmodium falciparum plasmepsin 2 expressed in Escherichia coli BL21 (DE3) after 40 mins by Kitz-Wilson plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 357nMAssay Description:Inhibition of Plasmodium falciparum plasmepsin 2More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Medical University Of South Carolina
Curated by ChEMBL
Medical University Of South Carolina
Curated by ChEMBL
Affinity DataKi: 385nMAssay Description:Competitive inhibition of recombinant LSD1 (unknown origin) using H3K4Me2 peptide as substrate preincubated for 30 mins followed by substrate additio...More data for this Ligand-Target Pair
TargetS-adenosylmethionine decarboxylase proenzyme(Rattus norvegicus)
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataKi: 560nMAssay Description:Inhibition of rat liver form of S-adenosyl-methionine decarboxylase enzymeMore data for this Ligand-Target Pair
TargetS-adenosylmethionine decarboxylase proenzyme(Homo sapiens (Human))
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of rat liver form of S-adenosyl-methionine decarboxylase enzymeMore data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Medical University Of South Carolina
Curated by ChEMBL
Medical University Of South Carolina
Curated by ChEMBL
Affinity DataKi: 1.80E+3nMAssay Description:Competitive inhibition of recombinant LSD1 (unknown origin) expressed in Escherichia coli BL21 (DE3) cells using H3K4 peptide as substrate by Dixon p...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Medical University Of South Carolina
Curated by ChEMBL
Medical University Of South Carolina
Curated by ChEMBL
Affinity DataKi: 2.20E+3nMAssay Description:Competitive inhibition of LSD1 (unknown origin) incubated for 30 mins using H3K4me2 substrate by fluorescence based assayMore data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Medical University Of South Carolina
Curated by ChEMBL
Medical University Of South Carolina
Curated by ChEMBL
Affinity DataKi: 2.40E+3nMAssay Description:Competitive inhibition of human recombinant LSD1 by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
TargetS-adenosylmethionine decarboxylase proenzyme(Homo sapiens (Human))
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataKi: 3.80E+3nMAssay Description:Compound was evaluated to inactivate the bacterial AdoMet-DC; value ranges from 3.8 to 39.6 uMMore data for this Ligand-Target Pair
TargetS-adenosylmethionine decarboxylase proenzyme(Homo sapiens (Human))
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataKi: 7.20E+3nMAssay Description:Compound was evaluated to inactivate the human AdoMet-DCMore data for this Ligand-Target Pair
TargetS-adenosylmethionine decarboxylase proenzyme(Homo sapiens (Human))
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataKi: 1.07E+4nMAssay Description:Compound was evaluated to inactivate the bacterial AdoMet-DC; value ranges from 3.8 to 39.6 uMMore data for this Ligand-Target Pair
TargetS-adenosylmethionine decarboxylase proenzyme(Homo sapiens (Human))
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataKi: 1.07E+4nMAssay Description:Compound was evaluated to inactivate the human AdoMet-DC; value ranges from 10.7 to 62.7 uMMore data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Medical University Of South Carolina
Curated by ChEMBL
Medical University Of South Carolina
Curated by ChEMBL
Affinity DataKi: 1.66E+4nMAssay Description:Time-dependent inhibition of recombinant LSD1 catalytic domain (178 to 831 amino acids) (unknown origin) expressed in baculovirus infected insect Sf9...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Medical University Of South Carolina
Curated by ChEMBL
Medical University Of South Carolina
Curated by ChEMBL
TargetS-adenosylmethionine decarboxylase proenzyme(Homo sapiens (Human))
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataKi: 1.80E+4nMAssay Description:Tested for irreversible inhibitory activity against AdoMet-DC (S-adenosyl-methionine decarboxylase-) isolated from Escherichia coliMore data for this Ligand-Target Pair
TargetS-adenosylmethionine decarboxylase proenzyme(Homo sapiens (Human))
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataKi: 2.12E+4nMAssay Description:compound was evaluated for the inhibitor constant against Escherichia coli S-adenosyl-L-methionine decarboxylaseMore data for this Ligand-Target Pair
TargetS-adenosylmethionine decarboxylase proenzyme(Homo sapiens (Human))
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataKi: 2.12E+4nMAssay Description:compound was evaluated for the inhibitor constant against human S-adenosyl-L-methionine decarboxylaseMore data for this Ligand-Target Pair
TargetS-adenosylmethionine decarboxylase proenzyme(Homo sapiens (Human))
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataKi: 2.12E+4nMAssay Description:compound was evaluated for the inhibitor constant against Escherichia coli S-adenosyl-L-methionine decarboxylaseMore data for this Ligand-Target Pair
TargetS-adenosylmethionine decarboxylase proenzyme(Homo sapiens (Human))
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataKi: 2.15E+4nMAssay Description:compound was evaluated for the inhibitor constant against Escherichia coli S-adenosyl-L-methionine decarboxylaseMore data for this Ligand-Target Pair
TargetS-adenosylmethionine decarboxylase proenzyme(Homo sapiens (Human))
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataKi: 2.37E+4nMAssay Description:Compound was evaluated for the inhibitor constant against Escherichia coli S-adenosyl-L-methionine decarboxylaseMore data for this Ligand-Target Pair
TargetS-adenosylmethionine decarboxylase proenzyme(Homo sapiens (Human))
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataKi: 2.37E+4nMAssay Description:Compound was evaluated for the inhibitor constant against Escherichia coli S-adenosyl-L-methionine decarboxylaseMore data for this Ligand-Target Pair
TargetS-adenosylmethionine decarboxylase proenzyme(Homo sapiens (Human))
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataKi: 3.11E+4nMAssay Description:Compound was evaluated to inactivate the bacterial AdoMet-DCMore data for this Ligand-Target Pair
Affinity DataKi: 5.00E+4nMAssay Description:Inhibitory activity against human SSATMore data for this Ligand-Target Pair
TargetS-adenosylmethionine decarboxylase proenzyme(Homo sapiens (Human))
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataKi: 9.52E+4nMAssay Description:compound was evaluated for the inhibitory constant against human S-adenosyl-L-methionine decarboxylaseMore data for this Ligand-Target Pair
TargetS-adenosylmethionine decarboxylase proenzyme(Homo sapiens (Human))
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataKi: 9.52E+4nMAssay Description:compound was evaluated for the inhibitory constant against human S-adenosyl-L-methionine decarboxylaseMore data for this Ligand-Target Pair
TargetS-adenosylmethionine decarboxylase proenzyme(Homo sapiens (Human))
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataKi: 2.47E+5nMAssay Description:Compound was evaluated to inactivate the human AdoMet-DCMore data for this Ligand-Target Pair
TargetS-adenosylmethionine decarboxylase proenzyme(Homo sapiens (Human))
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataKi: 4.15E+5nMAssay Description:Tested for irreversible inhibitory activity against AdoMet-DC (S-adenosyl-methionine decarboxylase-) isolated from Escherichia coliMore data for this Ligand-Target Pair
TargetS-adenosylmethionine decarboxylase proenzyme(Homo sapiens (Human))
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataKi: 4.78E+5nMAssay Description:Tested for irreversible inhibitory activity against AdoMet-DC (S-adenosyl-methionine decarboxylase-) isolated from Escherichia coliMore data for this Ligand-Target Pair
TargetS-adenosylmethionine decarboxylase proenzyme(Homo sapiens (Human))
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataKi: 6.69E+5nMAssay Description:Tested for irreversible inhibitory activity against AdoMet-DC (S-adenosyl-methionine decarboxylase-) isolated from Escherichia coliMore data for this Ligand-Target Pair
TargetS-adenosylmethionine decarboxylase proenzyme(Homo sapiens (Human))
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataKi: 9.60E+5nMAssay Description:Tested for irreversible inhibitory activity against AdoMet-DC (S-adenosyl-methionine decarboxylase-) isolated from Escherichia coliMore data for this Ligand-Target Pair
TargetS-adenosylmethionine decarboxylase proenzyme(Homo sapiens (Human))
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataKi: 1.07E+6nMAssay Description:Tested for irreversible inhibitory activity against AdoMet-DC (S-adenosyl-methionine decarboxylase-) isolated from Escherichia coliMore data for this Ligand-Target Pair
TargetS-adenosylmethionine decarboxylase proenzyme(Homo sapiens (Human))
Wayne State University
Curated by ChEMBL
Wayne State University
Curated by ChEMBL
Affinity DataKi: 1.07E+6nMAssay Description:Tested for irreversible inhibitory activity against AdoMet-DC (S-adenosyl-methionine decarboxylase-) isolated from Escherichia coliMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Inhibition of HIV-1 NL4-3 wild type protease expressed in Escherichia coli preincubated for 20 mins by FRET analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:Inhibition of HIV-1 NL4-3 wild type protease expressed in Escherichia coli preincubated for 20 mins by FRET analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.60nMAssay Description:Inhibition of HIV-1 NL4-3 wild type protease expressed in Escherichia coli preincubated for 20 mins by FRET analysisMore data for this Ligand-Target Pair
TargetHistone deacetylase 1(Homo sapiens (Human))
Medical University Of South Carolina
Curated by ChEMBL
Medical University Of South Carolina
Curated by ChEMBL
Affinity DataIC50: 14nMAssay Description:Inhibition of human recombinant HDAC1 by microplate reader assayMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Inhibitory activity against spermine synthaseMore data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Competitive inhibition of Plasmodium falciparum plasmepsin 2 expressed in Escherichia coli BL21 (DE3) after 30 mins by FRET assayMore data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)