Query String: CIDOFOVIR
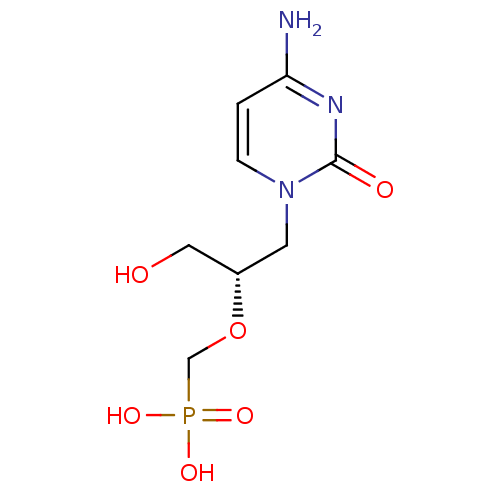 US10071110, Compound Cidofovir CDV HPMPC BDBM31915 Vistide Cidofovir
US10071110, Compound Cidofovir CDV HPMPC BDBM31915 Vistide Cidofovir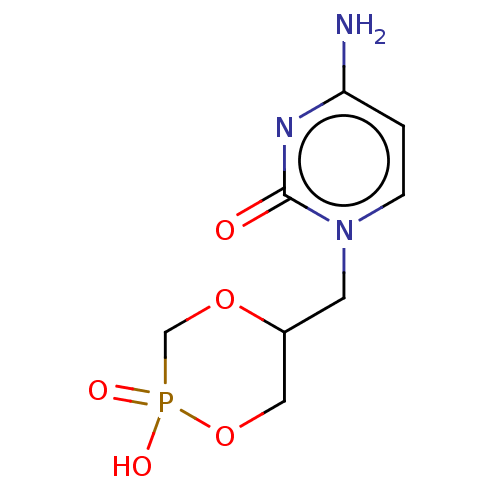 US10071110, Compound cCDV CYCLIC CIDOFOVIR US10071110, Compound Cyclic Cidofovir (cDCV) BDBM275768 Cyclic-hpmpc
US10071110, Compound cCDV CYCLIC CIDOFOVIR US10071110, Compound Cyclic Cidofovir (cDCV) BDBM275768 Cyclic-hpmpc BDBM275771 US10071110, Compound Cidofovir(CDV)
BDBM275771 US10071110, Compound Cidofovir(CDV) BDBM275772 US10071110, Compound Cyclic Cidofovir (cDCV)
BDBM275772 US10071110, Compound Cyclic Cidofovir (cDCV)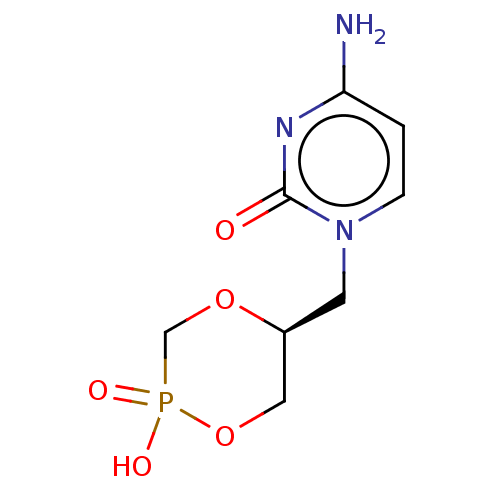 BDBM50485549 Cyclic Cidofovir Cyclic(S)-HPMPC
BDBM50485549 Cyclic Cidofovir Cyclic(S)-HPMPC US10071110, Compound CDV BDBM275767 US10071110, Compound Cidofovir(CDV)
US10071110, Compound CDV BDBM275767 US10071110, Compound Cidofovir(CDV) US10071110, Compound 1-O-hexadecylpropanediol-3-cCDV US10071110, Compound HDP-cCDV BDBM275769 Hexadecyloxypropyl-cyclic cidofovir
US10071110, Compound 1-O-hexadecylpropanediol-3-cCDV US10071110, Compound HDP-cCDV BDBM275769 Hexadecyloxypropyl-cyclic cidofovir
- CPE reduction assay The activity of cidofovir (CDV), cyclic cidofovir (cCDV), and 1-O-hexadecylpropanediol-3-cCDV (HDP-cCDV) were tested for antiviral activity in human foreskin fibroblasts infected with vaccinia virus or cowpox virus by measuring the dose dependent reduction in cytopathic effect (CPE). Preliminary vaccinia and cowpox EC50 values were determined in a CPE reduction assay in human foreskin fibroblast (HFF) cells.
- Viral Plaque Reduction Assay African green monkey kidney BSC-1 cells were grown in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (Gibco BRL Life Technologies, Gaithersburg, Md.) and 0.1% gentamicin antibiotic at 37° C. in a humidified 5% CO2 environment. Confluent BSC-1 cells were infected with vaccinia virus at an MOI of 0.005 in 48-well plate. The test compounds and control cidofovir were dissolved in DMSO and diluted with the medium. One hour post infection, 400 uL of the test compounds and control were added per well at concentrations ranging from 200 nM to 200 uM and incubated at 37° C. for 16 hours. A 5% solution of formaldehyde in PBS was used to fix the cells. After washing twice with PBS, the plate was stained with 0.2% crystal violet in 50% ethanol.