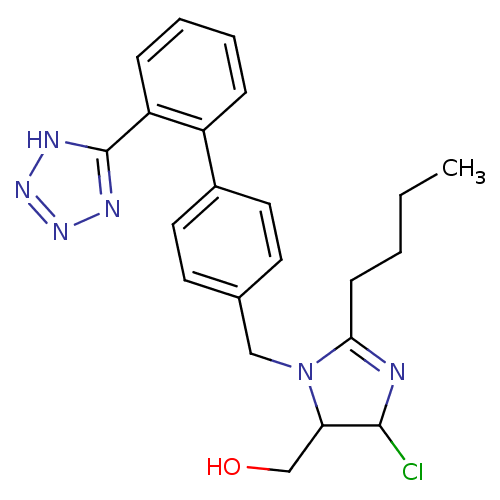
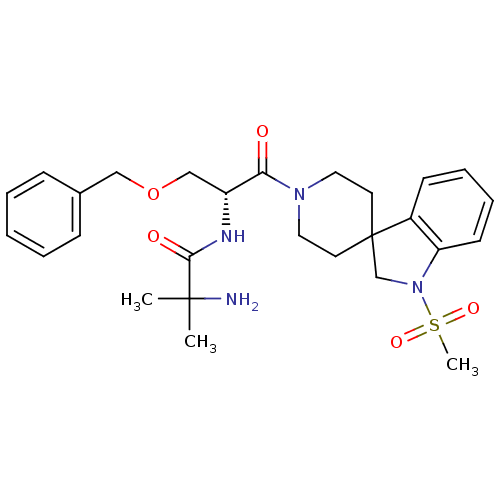
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Novo Nordisk
Curated by ChEMBL
Novo Nordisk
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of the compound against human growth hormone secretagogue (GHS) receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:Inhibition of the compound against human 5-hydroxytryptamine 6 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 28nMAssay Description:Inhibition of the compound against human angiotensin II receptor, type 1 (AG2-R)More data for this Ligand-Target Pair
Affinity DataIC50: 60nMAssay Description:Inhibition of the compound against rat growth hormone secretagogue (GHS) receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 63nMAssay Description:Inhibition of the compound against pig growth hormone secretagogue (GHS) receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of the compound against human melanocortin-4 (MC4) receptorMore data for this Ligand-Target Pair
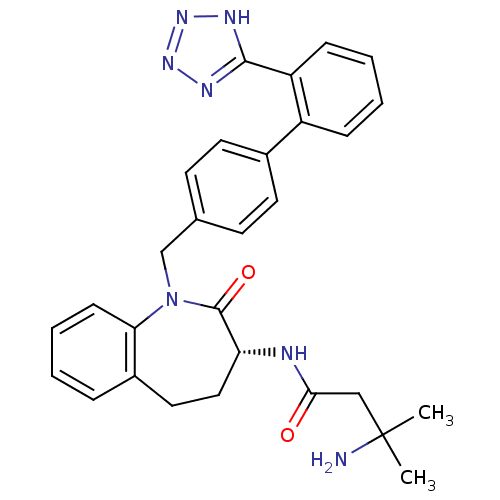
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Novo Nordisk
Curated by ChEMBL
Novo Nordisk
Curated by ChEMBL
Affinity DataIC50: 190nMAssay Description:Inhibition of the compound against human growth hormone secretagogue (GHS) receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 612nMAssay Description:Inhibition of the compound against human melanocortin-4 (MC4) receptorMore data for this Ligand-Target Pair
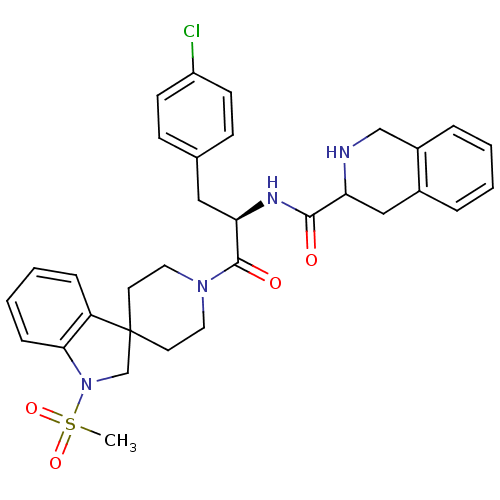
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Novo Nordisk
Curated by ChEMBL
Novo Nordisk
Curated by ChEMBL
Affinity DataIC50: 2.80E+3nMAssay Description:Inhibition of the compound against human growth hormone secretagogue (GHS) receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+3nMAssay Description:Inhibition of the compound against human angiotensin II receptor, type 1 (AG2-R)More data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)