Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Androgen receptor
Ligand
BDBM18163
Substrate
BDBM18161
Meas. Tech.
Receptor Binding and Transactivation Assay
pH
7.4±n/a
Temperature
295.15±n/a K
Ki
24±n/a nM
IC50
260±n/a nM
EC50
>1000±n/a nM
Citation
 Sun, C; Robl, JA; Wang, TC; Huang, Y; Kuhns, JE; Lupisella, JA; Beehler, BC; Golla, R; Sleph, PG; Seethala, R; Fura, A; Krystek, Sr; An, Y; Malley, MF; Sack, JS; Salvati, ME; Grover, GJ; Ostrowski, J; Hamann, LG Discovery of potent, orally-active, and muscle-selective androgen receptor modulators based on an N-aryl-hydroxybicyclohydantoin scaffold. J Med Chem 49:7596-9 (2006) [PubMed] Article
Sun, C; Robl, JA; Wang, TC; Huang, Y; Kuhns, JE; Lupisella, JA; Beehler, BC; Golla, R; Sleph, PG; Seethala, R; Fura, A; Krystek, Sr; An, Y; Malley, MF; Sack, JS; Salvati, ME; Grover, GJ; Ostrowski, J; Hamann, LG Discovery of potent, orally-active, and muscle-selective androgen receptor modulators based on an N-aryl-hydroxybicyclohydantoin scaffold. J Med Chem 49:7596-9 (2006) [PubMed] Article More Info.:
Target
Name:
Androgen receptor
Synonyms:
ANDR_HUMAN | AR | Androgen Receptor | Androgen receptor (AR) | Androgen receptor/Baculoviral IAP repeat-containing protein 2 | DHTR | Dihydrotestosterone receptor | NR3C4 | Nuclear receptor subfamily 3 group C member 4
Type:
Receptor
Mol. Mass.:
99185.27
Organism:
Homo sapiens (Human)
Description:
CHO cells were stably transfected with human AR gene.
Residue:
920
Sequence:
MEVQLGLGRVYPRPPSKTYRGAFQNLFQSVREVIQNPGPRHPEAASAAPPGASLLLLQQQQQQQQQQQQQQQQQQQQQQQETSPRQQQQQQGEDGSPQAHRRGPTGYLVLDEEQQPSQPQSALECHPERGCVPEPGAAVAASKGLPQQLPAPPDEDDSAAPSTLSLLGPTFPGLSSCSADLKDILSEASTMQLLQQQQQEAVSEGSSSGRAREASGAPTSSKDNYLGGTSTISDNAKELCKAVSVSMGLGVEALEHLSPGEQLRGDCMYAPLLGVPPAVRPTPCAPLAECKGSLLDDSAGKSTEDTAEYSPFKGGYTKGLEGESLGCSGSAAAGSSGTLELPSTLSLYKSGALDEAAAYQSRDYYNFPLALAGPPPPPPPPHPHARIKLENPLDYGSAWAAAAAQCRYGDLASLHGAGAAGPGSGSPSAAASSSWHTLFTAEEGQLYGPCGGGGGGGGGGGGGGGGGGGGGGGEAGAVAPYGYTRPPQGLAGQESDFTAPDVWYPGGMVSRVPYPSPTCVKSEMGPWMDSYSGPYGDMRLETARDHVLPIDYYFPPQKTCLICGDEASGCHYGALTCGSCKVFFKRAAEGKQKYLCASRNDCTIDKFRRKNCPSCRLRKCYEAGMTLGARKLKKLGNLKLQEEGEASSTTSPTEETTQKLTVSHIEGYECQPIFLNVLEAIEPGVVCAGHDNNQPDSFAALLSSLNELGERQLVHVVKWAKALPGFRNLHVDDQMAVIQYSWMGLMVFAMGWRSFTNVNSRMLYFAPDLVFNEYRMHKSRMYSQCVRMRHLSQEFGWLQITPQEFLCMKALLLFSIIPVDGLKNQKFFDELRMNYIKELDRIIACKRKNPTSCSRRFYQLTKLLDSVQPIARELHQFTFDLLIKSHMVSVDFPEMMAEIISVQVPKILSGKVKPIYFHTQ
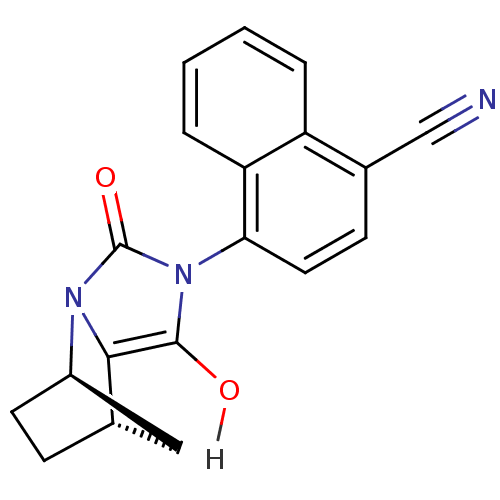
Inhibitor
Name:
BDBM18163
Synonyms:
4-[(1R,6R,7S)-3,5-dioxo-2,4-diazatricyclo[5.2.1.0^{2,6}]decan-4-yl]naphthalene-1-carbonitrile | N-aryl-bicyclic hydantoin, 4b
Type:
Small organic molecule
Emp. Form.:
C19H15N3O2
Mol. Mass.:
317.3413
SMILES:
Oc1c2[C@H]3CC[C@H](C3)n2c(=O)n1-c1ccc(C#N)c2ccccc12 |r,wU:6.6,3.7,(12.2,-7.28,;12.6,-8.77,;11.56,-9.96,;10.02,-9.96,;9.25,-11.29,;10.02,-12.62,;11.56,-12.62,;10.02,-11.73,;12.33,-11.29,;13.87,-10.92,;15.03,-11.93,;14,-9.4,;15.49,-9,;16.07,-7.57,;17.59,-7.36,;18.54,-8.57,;20.06,-8.36,;21.55,-7.96,;17.96,-10,;18.91,-11.21,;18.34,-12.64,;16.81,-12.86,;15.86,-11.64,;16.44,-10.22,)|
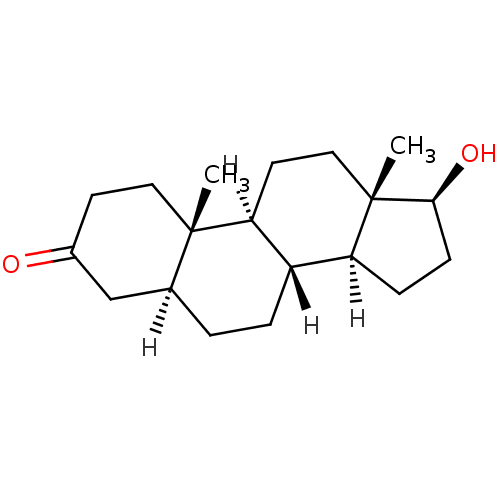
Substrate
Name:
BDBM18161
Synonyms:
(1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-5-one | (5alpha,17beta)-17-hydroxyandrostan-3-one | CHEMBL27769 | DHT | Dihydrotestosterone | [3H]DHT
Type:
Steroid
Emp. Form.:
C19H30O2
Mol. Mass.:
290.4403
SMILES:
[H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r|