Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cholecystokinin receptor type A
Ligand
BDBM50005452
Substrate
n/a
Meas. Tech.
ChEMBL_47647 (CHEMBL657359)
Ki
>2000±n/a nM
Citation
 van der Bent, A; Blommaert, AG; Melman, CT; IJzerman, AP; van Wijngaarden, I; Soudijn, W Hybrid cholecystokinin-A antagonists based on molecular modeling of lorglumide and L-364,718. J Med Chem 35:1042-9 (1992) [PubMed] Article
van der Bent, A; Blommaert, AG; Melman, CT; IJzerman, AP; van Wijngaarden, I; Soudijn, W Hybrid cholecystokinin-A antagonists based on molecular modeling of lorglumide and L-364,718. J Med Chem 35:1042-9 (1992) [PubMed] Article More Info.:
Target
Name:
Cholecystokinin receptor type A
Synonyms:
CCKAR_RAT | Cckar | Cholecystokinin peripheral | Cholecystokinin receptor | Cholecystokinin receptor type A
Type:
Enzyme Catalytic Domain
Mol. Mass.:
49676.37
Organism:
RAT
Description:
Cholecystokinin central 0 RAT::P30551
Residue:
444
Sequence:
MSHSPARQHLVESSRMDVVDSLLMNGSNITPPCELGLENETLFCLDQPQPSKEWQSALQILLYSIIFLLSVLGNTLVITVLIRNKRMRTVTNIFLLSLAVSDLMLCLFCMPFNLIPNLLKDFIFGSAVCKTTTYFMGTSVSVSTFNLVAISLERYGAICRPLQSRVWQTKSHALKVIAATWCLSFTIMTPYPIYSNLVPFTKNNNQTANMCRFLLPSDAMQQSWQTFLLLILFLLPGIVMVVAYGLISLELYQGIKFDASQKKSAKEKKPSTGSSTRYEDSDGCYLQKSRPPRKLELQQLSSGSGGSRLNRIRSSSSAANLIAKKRVIRMLIVIVVLFFLCWMPIFSANAWRAYDTVSAEKHLSGTPISFILLLSYTSSCVNPIIYCFMNKRFRLGFMATFPCCPNPGPPGVRGEVGEEEDGRTIRALLSRYSYSHMSTSAPPP
Inhibitor
Name:
BDBM50005452
Synonyms:
1H-Indole-2-carboxylic acid (4,4-diphenyl-but-3-enyl)-amide | CHEMBL267507
Type:
Small organic molecule
Emp. Form.:
C25H22N2O
Mol. Mass.:
366.455
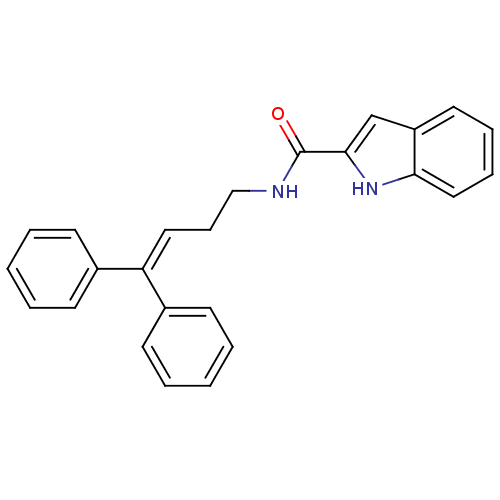
SMILES:
O=C(NCCC=C(c1ccccc1)c1ccccc1)c1cc2ccccc2[nH]1 |(21.68,-3.62,;22.45,-2.29,;21.7,-.96,;20.16,-.96,;19.4,.39,;17.86,.41,;17.1,1.74,;17.88,3.07,;17.13,4.4,;17.9,5.73,;19.44,5.72,;20.21,4.38,;19.42,3.05,;15.56,1.77,;14.82,3.1,;13.28,3.12,;12.49,1.79,;13.26,.44,;14.79,.44,;23.99,-2.31,;24.9,-1.07,;26.37,-1.55,;27.7,-.8,;29.01,-1.57,;29.01,-3.11,;27.67,-3.87,;26.37,-3.16,;24.88,-3.55,)|