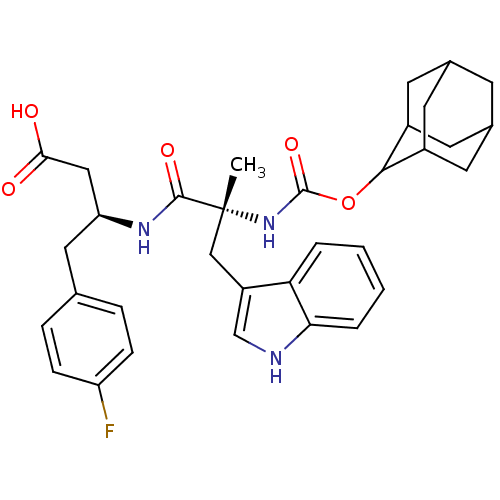
Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cholecystokinin receptor type A
Ligand
BDBM50451028
Substrate
n/a
Meas. Tech.
ChEBML_50044
IC50
75±n/a nM
Citation
 Bernad, N; Burgaud, BG; Horwell, DC; Lewthwaite, RA; Martinez, J; Pritchard, MC The design and synthesis of the high efficacy, non-peptide CCK1 receptor agonist PD170292. Bioorg Med Chem Lett 10:1245-8 (2000) [PubMed] Article
Bernad, N; Burgaud, BG; Horwell, DC; Lewthwaite, RA; Martinez, J; Pritchard, MC The design and synthesis of the high efficacy, non-peptide CCK1 receptor agonist PD170292. Bioorg Med Chem Lett 10:1245-8 (2000) [PubMed] Article More Info.:
Target
Name:
Cholecystokinin receptor type A
Synonyms:
CCKAR_RAT | Cckar | Cholecystokinin peripheral | Cholecystokinin receptor | Cholecystokinin receptor type A
Type:
Enzyme Catalytic Domain
Mol. Mass.:
49676.37
Organism:
RAT
Description:
Cholecystokinin central 0 RAT::P30551
Residue:
444
Sequence:
MSHSPARQHLVESSRMDVVDSLLMNGSNITPPCELGLENETLFCLDQPQPSKEWQSALQILLYSIIFLLSVLGNTLVITVLIRNKRMRTVTNIFLLSLAVSDLMLCLFCMPFNLIPNLLKDFIFGSAVCKTTTYFMGTSVSVSTFNLVAISLERYGAICRPLQSRVWQTKSHALKVIAATWCLSFTIMTPYPIYSNLVPFTKNNNQTANMCRFLLPSDAMQQSWQTFLLLILFLLPGIVMVVAYGLISLELYQGIKFDASQKKSAKEKKPSTGSSTRYEDSDGCYLQKSRPPRKLELQQLSSGSGGSRLNRIRSSSSAANLIAKKRVIRMLIVIVVLFFLCWMPIFSANAWRAYDTVSAEKHLSGTPISFILLLSYTSSCVNPIIYCFMNKRFRLGFMATFPCCPNPGPPGVRGEVGEEEDGRTIRALLSRYSYSHMSTSAPPP
Inhibitor
Name:
BDBM50451028
Synonyms:
CHEMBL2062146 | PD-149164
Type:
Small organic molecule
Emp. Form.:
C33H38FN3O5
Mol. Mass.:
575.6703
SMILES:
C[C@](Cc1c[nH]c2ccccc12)(NC(=O)OC1C2CC3CC(C2)CC1C3)C(=O)N[C@H](CC(O)=O)Cc1ccc(F)cc1 |wD:29.34,1.0,TLB:18:19:23:16.17.22,15:16:23:19.25.20,THB:18:17:23:19.25.20,20:19:16:21.23.22,20:21:16:19.18.25,(7.6,-.86,;7.7,-2.9,;7.7,-4.44,;6.91,-5.77,;7.73,-7.1,;6.7,-8.26,;5.28,-7.66,;3.9,-8.29,;2.62,-7.43,;2.76,-5.89,;4.18,-5.23,;5.41,-6.12,;6.37,-2.13,;5.02,-2.9,;5.02,-4.44,;3.69,-2.12,;2.36,-2.89,;2.36,-4.43,;1.57,-5.75,;.24,-4.98,;-1.39,-5.51,;-.3,-4.43,;1.03,-5.19,;-.3,-2.89,;1.03,-2.11,;.26,-3.44,;9.03,-2.13,;9.03,-.59,;10.36,-2.9,;11.7,-2.12,;11.7,-.58,;13.03,.19,;14.52,.6,;13.03,1.73,;13.03,-2.89,;14.37,-2.12,;15.7,-2.89,;17.03,-2.12,;17.03,-.58,;18.36,.2,;15.68,.19,;14.35,-.59,)|