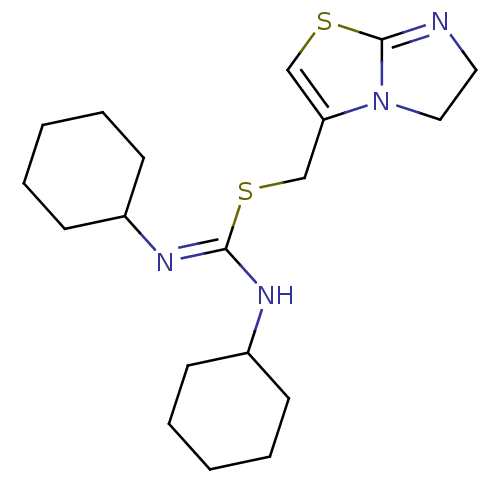
BDBM50246956 1,3-dicyclohexyl-2-((5,6-dihydroimidazo[2,1-b]thiazol-3-yl)methyl)isothiourea::CHEMBL452864
SMILES C(S\C(NC1CCCCC1)=N/C1CCCCC1)C1=CSC2=NCCN12
InChI Key InChIKey=ZEZPDHKACVMMCD-UHFFFAOYSA-N
Data 13 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 13 hits for monomerid = 50246956
Found 13 hits for monomerid = 50246956
TargetC-X-C chemokine receptor type 4(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Activity at CXCR4 in human CEM cells assessed as inhibition of CXCL12-induced calcium mobilizationMore data for this Ligand-Target Pair
TargetC-X-C chemokine receptor type 4(Rat)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 29nMAssay Description:Activity at CXCR4 in rat IR983F cells assessed as inhibition of CXCL12-induced cell migrationMore data for this Ligand-Target Pair
TargetC-X-C chemokine receptor type 4(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 48nMAssay Description:Displacement of [125I]CXCL12 from CXCR4 in human CEM cellsMore data for this Ligand-Target Pair
TargetC-X-C chemokine receptor type 4(Rat)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 55nMAssay Description:Displacement of [125I]CXCL12 from CXCR4 in rat IR983F cellsMore data for this Ligand-Target Pair
TargetC-X-C chemokine receptor type 4(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 58nMAssay Description:Activity at CXCR4 in human Jurkat T cells assessed as inhibition of CXCL12-induced cell migrationMore data for this Ligand-Target Pair
TargetC-C chemokine receptor type 7(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of CCR7More data for this Ligand-Target Pair
Affinity DataIC50: 3.86E+3nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair
Affinity DataIC50: 6.78E+3nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 8.50E+3nMAssay Description:Displacement of [3H]dofetilide from human recombinant ERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CYP2C19More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of human recombinant ERG in CHOK1 cellsMore data for this Ligand-Target Pair