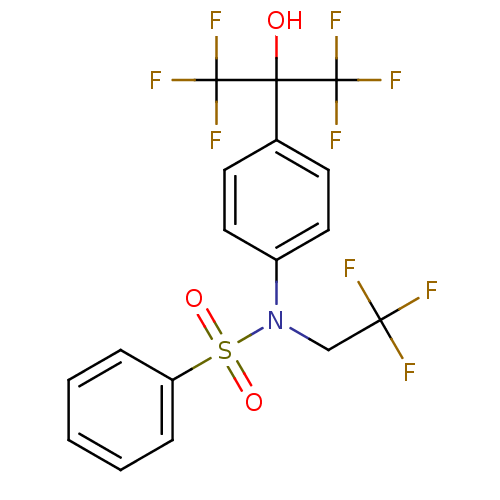
BDBM19993 CHEMBL62136::N-[4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)phenyl]-N-(2,2,2-trifluoroethyl)benzenesulfonamide::T 0901317::T0901317::TO-901317::US10543183, Compound TO901317::US10669296, Compound TO901317::US10945978, Compound 1::US20250282736, Compound T0901317::[3H]T0901317
SMILES c1ccc(cc1)S(=O)(=O)[N@@](CC(F)(F)F)c2ccc(cc2)C(C(F)(F)F)(C(F)(F)F)O
InChI Key InChIKey=SGIWFELWJPNFDH-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 180 hits for monomerid = 19993
Found 180 hits for monomerid = 19993
TargetOxysterols receptor LXR-alpha/Retinoic acid receptor RXR-alpha(Human)
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
Affinity DataEC50: 0.0980nMAssay Description:Agonist activity at human RXRalpha/LXRalpha expressed in HEK293 cells measured after 20 hrs by dual luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-beta/Retinoic acid receptor RXR-alpha(Human)
Sun Yat-Sen University
Curated by ChEMBL
Sun Yat-Sen University
Curated by ChEMBL
Affinity DataEC50: 0.108nMAssay Description:Agonist activity at human RXRalpha/LXRbeta expressed in HEK293 cells measured after 20 hrs by dual luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: 3.20nMAssay Description:Agonist activity at LXRalpha by TR-FRET assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-beta(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: 4.70nMAssay Description:Agonist activity at LXRbeta by TR-FRET assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: 6nMAssay Description:Agonist activity at human LXRalpha expressed in HEK293 cells co-expressing CMX-beta-galactosidase incubated for 16 hrs by luciferase reporter gene as...More data for this Ligand-Target Pair
Affinity DataEC50: 8nMAssay Description:Transactivation of PXR in human HepG2 cells by receptor transactivation assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-beta(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: 8nMAssay Description:Agonist activity at LXRbeta ligand binding domain by FRET based SRC1 recruitment assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRalpha LBDMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-beta(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Displacement of [3H]T0901317 from LXRbeta ligand binding domainMore data for this Ligand-Target Pair
Affinity DataIC50: 9nM EC50: 170nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
TargetOxysterols receptor LXR-beta(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Displacement of [3H]TO901317 from human recombinant LXRbeta expressed in Escherichia coli by flashplate methodMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRalpha LBDMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-beta(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Binding affinity at human LXRbetaMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRalpha-LBDMore data for this Ligand-Target Pair
Affinity DataIC50: 9nM EC50: 16nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ...More data for this Ligand-Target Pair
TargetOxysterols receptor LXR-beta(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Binding affinity to human recombinant LXRbeta ligand binding domainMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: 10nMAssay Description:Activity against LXR alpha transiently transfected in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 10nM EC50: 16nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: 10nMAssay Description:Agonist activity at LXRalpha ligand binding domain by FRET based SRC1 recruitment assayMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
TargetOxysterols receptor LXR-beta(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: 10nMAssay Description:Agonist activity at human LXR-betaMore data for this Ligand-Target Pair
Affinity DataIC50: 13nM EC50: 140nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Displacement of [3H]T0901317 from LXRalpha ligand binding domainMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Binding affinity at human LXRalphaMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Displacement of [3H]T0901317 from human LXRalpha ligand binding domainMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-beta(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRbeta-LBDMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-beta(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRbeta LBDMore data for this Ligand-Target Pair
Affinity DataIC50: 40nM EC50: 13nMpH: 8.0 T: 2°CAssay Description:Polylysine YiO imaging beads (Amersham, GE Healthcare) were coated with histidine-tagged WT PXR LBD. Nonspecific binding sites were blocked with BSA....More data for this Ligand-Target Pair
TargetOxysterols receptor LXR-beta(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Displacement of [3H]T0901317 from human recombinant LXRbeta LBDMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Displacement of [3H]TO901317 from human recombinant LXRalpha expressed in Escherichia coli by flashplate methodMore data for this Ligand-Target Pair
Affinity DataIC50: 13nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXRbeta coated flash plates. Each concentration of ...More data for this Ligand-Target Pair
TargetOxysterols receptor LXR-beta(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: 15nMAssay Description:Polyhistidine-tagged human LXR ligand-binding domain was mixed with the test compound, biotin-SRC1 peptide, streptavidin-allophycocyanin, and europiu...More data for this Ligand-Target Pair
Affinity DataEC50: 15nMAssay Description:Agonist activity at His-tagged PXR-LBD/SRC-1p (unknown origin) expressed in Escherichia coli BL21(DE3) by coactivator recruitment based Alpha-screen ...More data for this Ligand-Target Pair
TargetOxysterols receptor LXR-beta(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataKi: 17nMAssay Description:Displacement of radiolabeled T0901317 from LXRbeta LBD (unknown origin)More data for this Ligand-Target Pair
TargetOxysterols receptor LXR-beta(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: 19nMAssay Description:Agonist activity at human LXRbeta in HEK293 cells assessed as Gal4 transactivationMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-beta(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataIC50: 20nMAssay Description:Displacement of [N-methyl-3H]T1317 from human biotinylated LXRbeta LBD after 3 hrs by LEAD seeker binding assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: 20nMAssay Description:Agonist activity at LXRalpha (unknown origin) expressed in human HepG2 cells co-expressing ABCA1 promoter measured after 48 hrs by cell-based transac...More data for this Ligand-Target Pair
TargetOxysterols receptor LXR-beta(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: 20nMAssay Description:Agonist activity at LXRbeta (unknown origin) expressed in human H4 cells co-expressing ABCA1 promoter measured after 48 hrs by cell-based transactiva...More data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
TargetOxysterols receptor LXR-alpha(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: 20nMAssay Description:As used herein, reference to the activity of an LXR agonist at LXRα and LXRβ refer to the activity as measured using the ligand sensing ass...More data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: 20nMAssay Description:As used herein, reference to the activity of an LXR agonist at LXRα and LXRβ refer to the activity as measured using the ligand sensing ass...More data for this Ligand-Target Pair
Affinity DataEC50: 20nMAssay Description:Agonist activity at SREBP1c (unknown origin) stably transfected in human HepG2 cells assessed as increase in luciferase activity by counterscreen luc...More data for this Ligand-Target Pair
TargetOxysterols receptor LXR-beta(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: 21nMAssay Description:Agonist activity at human LXRbeta expressed in HEK293 cells co-expressing CMX-beta-galactosidase incubated for 16 hrs by luciferase reporter gene ass...More data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataKi: 24nMAssay Description:Displacement of radiolabeled T0901317 from LXRalpha LBD (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 26nMAssay Description:Inhibition of BODIPY FL vindoline binding to recombinant human GST-tagged PXR LBD (111 to 434 residues) expressed in baculovirus infected insect cell...More data for this Ligand-Target Pair
Affinity DataIC50: 26nMAssay Description:PXR can be determined in an in vitro assay system. Such in vitro assay systems include assay such as the assays as described herein.More data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: 28nMAssay Description:Transactivation of LXRalpha (unknown origin) expressed in CV1 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: 30nMAssay Description:Agonist activity at human LXRalpha expressed in African green monkey kidney CV1 cells co-expressing pTAL-LXRE including LXR response element incubate...More data for this Ligand-Target Pair
TargetOxysterols receptor LXR-beta(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataEC50: 30nMpH: 7.5 T: 2°CAssay Description:The LXR LiSA measures the ligand-dependent recruitment of a 25 amino acid fragment of the steroid receptor coactivator 1 (SRC1) to the ligand-binding...More data for this Ligand-Target Pair
TargetOxysterols receptor LXR-beta(Rat)
Niigata University of Pharmacy and Applied Life Sciences
Curated by ChEMBL
Niigata University of Pharmacy and Applied Life Sciences
Curated by ChEMBL
Affinity DataEC50: 30nMAssay Description:Transactivation of rat LXRbeta expressed in HEK293FT cells measured after 14 to 18 hrs by dual-luciferase reporter gene assayMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)