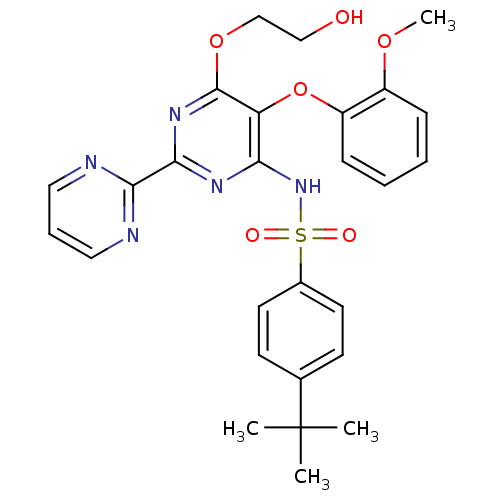
BDBM50061101 4-(1,1-Dimethylethyl)-N-(6-(2-hydroxyethoxy)-5-(2-methoxyphenoxy)-(2,2'-bipyrimidin)-4-yl) benzenesulfornamide::4-tert-butyl-N-[6-(2-hydroxyethoxy)-5-(2-methoxyphenoxy)-2,2'-bipyrimidin-4-yl]benzenesulfonamide::BOSENTAN::CHEMBL957::p-tert-Butyl-N-(6-(2-hydroxyethoxy)-5-(o-methoxyphenoxy)-2-(2-pyrimidinyl)-4-pyrimidinyl)benzenesulfonamide
SMILES CC(C)(C)c1ccc(cc1)S(=O)(=O)Nc2c(c(nc(n2)c3ncccn3)OCCO)Oc4ccccc4OC
InChI Key InChIKey=GJPICJJJRGTNOD-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 51 hits for monomerid = 50061101
Found 51 hits for monomerid = 50061101
Affinity DataKi: 3.70nMAssay Description:Displacement of [125I]endhothelin-1 from human ETA receptor stably expressed in CHO cells Membrane assessed as inhibition constant at 25 degC incubat...More data for this Ligand-Target Pair
Affinity DataIC50: 4.70nMAssay Description:Displacement of [125I]ET-1 from rat ETA receptor after 1 hr by Lowry methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.70nMAssay Description:Binding affinity to human ETA receptorMore data for this Ligand-Target Pair
Affinity DataKi: 6.5nMAssay Description:Binding affinity towards Endothelin A receptorMore data for this Ligand-Target Pair
Affinity DataKi: 6.5nMAssay Description:Binding affinity to ETA receptor (unknown origin) assessed as inhibition constantMore data for this Ligand-Target Pair
Affinity DataKi: 6.5nMAssay Description:Ability to displace endothelin ([125I]ET1) from human Endothelin A receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 7.5nMAssay Description:In vitro inhibition of [125 I]ET-1 binding to Endothelin A receptor in porcine aortic membraneMore data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:Inhibitory activity against human endothelin A receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 8.90nMAssay Description:Antagonist activity at human endothelin receptor subtype A expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:In vitro inhibitory concentration required against [125I]ET1 binding to membranes of CHO cells expressing human ETA receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 45nMAssay Description:Displacement of [I125]ET1 from recombinant ETA receptor expressed in CHO cells after 2 hrs by TopCount analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 45nMAssay Description:Displacement of 125I-ET1 from human smooth muscle ETA receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 45nMAssay Description:Displacement of 125I-ET1 from human smooth muscle ETA receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 45nMAssay Description:Antagonist activity at ET-A receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:Displacement of radioligand from ETA receptor (unknown origin) expressed in fall armyworm sf9 cell membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:Receptor binding affinity was determined in a radioligand binding assay against [125I]ET1 with recombinant human ETA receptor, expressed in baculovir...More data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:Receptor binding affinity was determined against [125I]ET1 with recombinant human ETA receptor, expressed in baculovirus-infected Sf9 cellsMore data for this Ligand-Target Pair
TargetEndothelin receptor type B(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 80nMAssay Description:Inhibitory activity against human endothelin B receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:Displacement of radioligand from ETA receptor (unknown origin) expressed in fall armyworm sf9 cell membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:Binding affinity to recombinant human ETAMore data for this Ligand-Target Pair
TargetEndothelin receptor type B(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 95nMAssay Description:Binding affinity to human ETB receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 95nMAssay Description:Displacement of [125I]ET-1 from rat ETB receptor after 1 hr by Lowry methodMore data for this Ligand-Target Pair
TargetEndothelin receptor type B(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
TargetEndothelin receptor type B(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
TargetEndothelin receptor type B(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 150nMAssay Description:Displacement of radioligand from ETB receptor in human placenta cell membranesMore data for this Ligand-Target Pair
TargetEndothelin receptor type B(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 150nMAssay Description:Displacement of radioligand from ETB receptor in human placenta cell membranesMore data for this Ligand-Target Pair
TargetEndothelin receptor type B(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 150nMAssay Description:Receptor binding affinity was determined against [125I]ET1 with membranes prepared from human placenta for ETB receptorMore data for this Ligand-Target Pair
TargetEndothelin receptor type B(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 202nMAssay Description:Antagonist activity at ET-B receptor (unknown origin)More data for this Ligand-Target Pair
TargetEndothelin receptor type B(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 202nMAssay Description:Displacement of 125I-ET1 from human placenta ETB receptorMore data for this Ligand-Target Pair
TargetEndothelin receptor type B(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 202nMAssay Description:Displacement of 125I-ET1 from human placenta ETB receptorMore data for this Ligand-Target Pair
TargetEndothelin receptor type B(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 202nMAssay Description:Displacement of [I125]ET1 from recombinant ETB receptor expressed in CHO cells after 2 hrs by TopCount analysisMore data for this Ligand-Target Pair
TargetEndothelin receptor type B(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 280nMAssay Description:In vitro inhibitory concentration required against [125I]ET1 binding to membranes of CHO cells expressing human ETB receptorMore data for this Ligand-Target Pair
TargetEndothelin receptor type B(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 340nMAssay Description:Ability to displace endothelin ([125I]ET1) from human Endothelin B receptorMore data for this Ligand-Target Pair
TargetEndothelin receptor type B(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 343nMAssay Description:Binding affinity to ETB receptor (unknown origin) assessed as inhibition constantMore data for this Ligand-Target Pair
TargetEndothelin receptor type B(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 423nMAssay Description:Displacement of [125I]endhothelin-3 from human ETB receptor stably expressed in CHO cells Membrane assessed as inhibition constant at 25 degC incubat...More data for this Ligand-Target Pair
TargetEndothelin receptor type B(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Antagonist activity at ETB receptor (unknown origin) assessed as effect on G protein-mediated smooth muscle contractionMore data for this Ligand-Target Pair
Affinity DataIC50: 1.59E+3nMAssay Description:Antagonist activity at ETA receptor (unknown origin) assessed as increase in G protein-mediated vasoconstrictionMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Human)
National Institutes of Health Chemical Genomics Center
Curated by ChEMBL
National Institutes of Health Chemical Genomics Center
Curated by ChEMBL
Affinity DataEC50: 8.90E+3nMAssay Description:Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.20E+4nMAssay Description:TP_TRANSPORTER: inhibition of Taurocholate uptake in membrane vesicle from Bsep-expressing Sf9-cellMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Human)
National Institutes of Health Chemical Genomics Center
Curated by ChEMBL
National Institutes of Health Chemical Genomics Center
Curated by ChEMBL
Affinity DataEC50: 1.26E+4nMAssay Description:Activation of human PXR expressed in human HepG2 (DPX-2) cells assessed as induction of CYP3A4 after 24 hrs by luminescent analysisMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Human)
National Institutes of Health Chemical Genomics Center
Curated by ChEMBL
National Institutes of Health Chemical Genomics Center
Curated by ChEMBL
Affinity DataEC50: 1.41E+4nMAssay Description:Activation of human PXR expressed in human HepG2 (DPX-2) cells after 24 hrs by luciferase reporter gene based luminescent analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+4nMAssay Description:Inhibition of human BSEP expressed in fall armyworm sf9 cell plasma membrane vesicles assessed as reduction in vesicle-associated [3H]-taurocholate t...More data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+4nMAssay Description:Inhibition of human MRP4 overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ATP and...More data for this Ligand-Target Pair
Affinity DataIC50: 2.30E+4nMAssay Description:Inhibition of human BSEP overexpressed in Sf9 cell membrane vesicles assessed as uptake of [3H]-taurocholate in presence of ATP measured after 15 to ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.53E+4nMAssay Description:Inhibition of human BSEP expressed in baculovirus transfected fall armyworm Sf21 cell membranes vesicles assessed as reduction in ATP-dependent [3H]-...More data for this Ligand-Target Pair
Affinity DataIC50: 2.90E+4nMAssay Description:Inhibition of recombinant human BSEP expressed in baculovirus infected sf9 cell membrane vesicles assessed as reduction in ATP or AMP-dependent [3H]-...More data for this Ligand-Target Pair
Affinity DataIC50: 3.06E+4nMAssay Description:Inhibition of Sprague-Dawley rat Bsep expressed in plasma membrane vesicles of Sf21 cells assessed as inhibition of ATP-dependent [3H]taurocholate up...More data for this Ligand-Target Pair
Affinity DataIC50: 3.81E+4nMAssay Description:Inhibition of human BSEP expressed in plasma membrane vesicles of Sf21 cells assessed as inhibition of ATP-dependent [3H]taurocholate uptakeMore data for this Ligand-Target Pair
Affinity DataIC50: 4.20E+4nMAssay Description:Inhibition of human MRP3 overexpressed in Sf9 insect cell membrane vesicles assessed as uptake of [3H]-estradiol-17beta-D-glucuronide in presence of ...More data for this Ligand-Target Pair
Affinity DataIC50: 6.08E+4nMAssay Description:Inhibition of DHPS (unknown origin ) incubated for 30 mins by NAD/NADH-Glow assayMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)