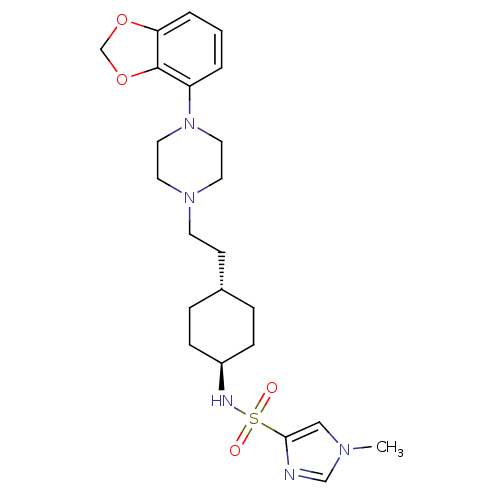
BDBM121522 US8722683, 69
SMILES Cn1cnc(c1)S(=O)(=O)N[C@H]1CC[C@H](CCN2CCN(CC2)c2cccc3OCOc23)CC1
InChI Key InChIKey=BYGFRAXTFHIMHT-WGSAOQKQSA-N
Data 3 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 121522
Found 3 hits for monomerid = 121522
Affinity DataKi: 2.25nMAssay Description:Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl...More data for this Ligand-Target Pair
Affinity DataKi: 55.3nMAssay Description:Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl...More data for this Ligand-Target Pair
Affinity DataKi: 68.0nMAssay Description:Aliquots of membrane preparations were thawed at RT, resupended in assay buffer (D2, D3: 50 mM Tris-HCl, 120 mM NaCl, 5 mM MgCl2, 1 mM EDTA, 5 mM KCl...More data for this Ligand-Target Pair