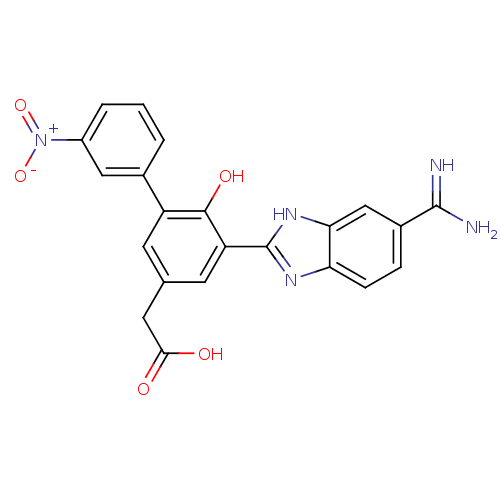
BDBM13776 2-[3-(5-carbamimidoyl-1H-1,3-benzodiazol-2-yl)-4-hydroxy-5-(3-nitrophenyl)phenyl]acetic acid::CHEMBL207720::amidine-containing inhibitor 1
SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(CC(O)=O)cc(c1O)-c1cccc(c1)[N+]([O-])=O
InChI Key InChIKey=RQCZRRWVWILIAI-UHFFFAOYSA-N
Data 5 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 13776
Found 5 hits for monomerid = 13776
Affinity DataKi: 15nM ΔG°: -10.6kcal/molepH: 7.4 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 15nMAssay Description:Binding affinity to factor7a/TF complexMore data for this Ligand-Target Pair
Affinity DataKi: 320nM ΔG°: -8.77kcal/molepH: 7.4 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: 1.10E+4nM ΔG°: -6.69kcal/molepH: 7.4 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair
Affinity DataKi: >1.50E+5nM ΔG°: >-5.16kcal/molepH: 7.4 T: 2°CAssay Description:The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su...More data for this Ligand-Target Pair