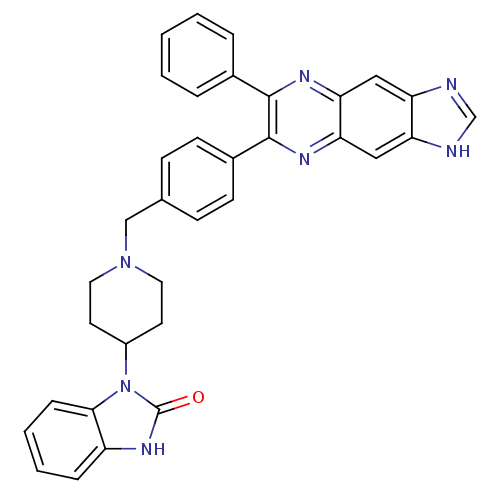
BDBM15169 1-{1-[(4-{7-phenyl-1H-imidazo[4,5-g]quinoxalin-6-yl}phenyl)methyl]piperidin-4-yl}-2,3-dihydro-1H-1,3-benzodiazol-2-one::3-[1-[4-(7-phenyl-3H-imidazo[4,5-g]quinoxalin-6-yl)benzyl]-4-piperidyl]-1H-benzimidazol-2-one::imidazoquinoxaline::imidazoquinoxaline 16h
SMILES O=c1[nH]c2ccccc2n1C1CCN(Cc2ccc(cc2)-c2nc3cc4[nH]cnc4cc3nc2-c2ccccc2)CC1
InChI Key InChIKey=BIWGYFZAEWGBAL-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 23 hits for monomerid = 15169
Found 23 hits for monomerid = 15169
Affinity DataIC50: 58nMpH: 7.5 T: 2°CAssay Description:Purified recombinant human Akt1, Akt2, and Akt3 proteins were monitored for kinase activity in the presence or absence of Akt inhibitors in a 96-well...More data for this Ligand-Target Pair
Affinity DataIC50: 2.40E+4nMAssay Description:Inhibition of RUVBL1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.12E+3nMAssay Description:Purified recombinant human Akt1, Akt2, and Akt3 proteins were monitored for kinase activity in the presence or absence of Akt inhibitors in a 96-well...More data for this Ligand-Target Pair
TargetLow molecular weight phosphotyrosine protein phosphatase(Homo sapiens (Human))
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
TargetLow molecular weight phosphotyrosine protein phosphatase(Homo sapiens (Human))
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 2.20E+3nMAssay Description:Inhibition of Akt3 (unknown origin)More data for this Ligand-Target Pair
TargetRAC-beta serine/threonine-protein kinase(Homo sapiens (Human))
University Of Bordeaux
Curated by ChEMBL
University Of Bordeaux
Curated by ChEMBL
Affinity DataIC50: 10nMAssay Description:Inhibition of Akt2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 58nMAssay Description:Inhibition of Akt1 (unknown origin)More data for this Ligand-Target Pair
TargetRAC-beta serine/threonine-protein kinase(Homo sapiens (Human))
University Of Bordeaux
Curated by ChEMBL
University Of Bordeaux
Curated by ChEMBL
TargetRAC-beta serine/threonine-protein kinase(Homo sapiens (Human))
University Of Bordeaux
Curated by ChEMBL
University Of Bordeaux
Curated by ChEMBL
TargetRAC-beta serine/threonine-protein kinase(Homo sapiens (Human))
University Of Bordeaux
Curated by ChEMBL
University Of Bordeaux
Curated by ChEMBL
Affinity DataIC50: 900nMAssay Description:Inhibition of Akt2 by cell-based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Inhibition of Akt1 by cell-based assayMore data for this Ligand-Target Pair
Affinity DataIC50: 58nMAssay Description:Inhibition of human recombinant Akt1More data for this Ligand-Target Pair
TargetRAC-beta serine/threonine-protein kinase(Homo sapiens (Human))
University Of Bordeaux
Curated by ChEMBL
University Of Bordeaux
Curated by ChEMBL
Affinity DataIC50: 210nMAssay Description:Inhibition of human recombinant Akt2More data for this Ligand-Target Pair
Affinity DataIC50: >2.12E+3nMAssay Description:Inhibition of human recombinant Akt3More data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+3nMAssay Description:Inhibition of Akt3More data for this Ligand-Target Pair
TargetRAC-beta serine/threonine-protein kinase(Homo sapiens (Human))
University Of Bordeaux
Curated by ChEMBL
University Of Bordeaux
Curated by ChEMBL
Affinity DataIC50: 305nMAssay Description:Inhibition of Akt1 by cell-based IPKA assayMore data for this Ligand-Target Pair
TargetRAC-beta serine/threonine-protein kinase(Homo sapiens (Human))
University Of Bordeaux
Curated by ChEMBL
University Of Bordeaux
Curated by ChEMBL
Affinity DataIC50: 2.10E+3nMAssay Description:Inhibition of Akt2 by cell-based IPKA assayMore data for this Ligand-Target Pair
TargetRAC-beta serine/threonine-protein kinase(Homo sapiens (Human))
University Of Bordeaux
Curated by ChEMBL
University Of Bordeaux
Curated by ChEMBL
Affinity DataIC50: 210nMAssay Description:Purified recombinant human Akt1, Akt2, and Akt3 proteins were monitored for kinase activity in the presence or absence of Akt inhibitors in a 96-well...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)