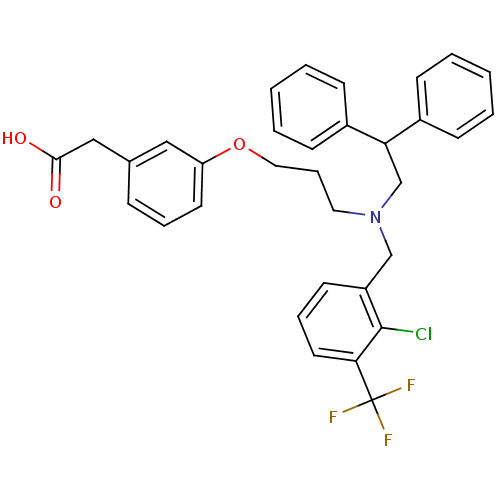
BDBM19992 2-[3-[3-[[2-chloro-3-(trifluoromethyl)phenyl]methyl-[2,2-di(phenyl)ethyl]amino]propoxy]phenyl]acetic acid::2-{3-[3-({[2-chloro-3-(trifluoromethyl)phenyl]methyl}(2,2-diphenylethyl)amino)propoxy]phenyl}acetic acid::CHEMBL59030::GSK-3965::GW3965::US10543183, Compound GW3965::US10669296, Compound GW3965::US10945978, Compound 2
SMILES OC(=O)Cc1cccc(OCCCN(CC(c2ccccc2)c2ccccc2)Cc2cccc(c2Cl)C(F)(F)F)c1
InChI Key InChIKey=NAXSRXHZFIBFMI-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 14 hits for monomerid = 19992
Found 14 hits for monomerid = 19992
Affinity DataIC50: 121nMAssay Description:SPA assays were run in displacement mode using 30 nM [3H]-GW3965. Ligands were tested in 12-point dose-response curves starting at 20 uM. IC50 values...More data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:SPA assays were run in displacement mode using 30 nM [3H]-GW3965. Ligands were tested in 12-point dose-response curves starting at 20 uM. IC50 values...More data for this Ligand-Target Pair
Affinity DataIC50: 4.80E+3nMpH: 7.0 T: 2°CAssay Description:The alpha-glucosidase inhibitory activity of test compounds was determined in a 96-well plate format. The reaction mixture containing enzyme and chro...More data for this Ligand-Target Pair
Affinity DataIC50: 12nM EC50: 310nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 100nM EC50: 660nMAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRbeta ligand binding domainMore data for this Ligand-Target Pair
Affinity DataIC50: 7.90nMAssay Description:Displacement of [N-methyl-3H]T1317 from human biotinylated LXRbeta LBD after 3 hrs by LEAD seeker binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Antagonist activity at LXRbeta ligand binding domain assessed as inhibition of T1317-induced transcriptional activity in african green monkey CV1 cel...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Antagonist activity at LXRalpha ligand binding domain assessed as inhibition of T1317-induced transcriptional activity in african green monkey CV1 ce...More data for this Ligand-Target Pair
Affinity DataIC50: 235nMAssay Description:Displacement of [N-methyl-3H]GW0438 from human biotinylated LXRalpha ligand binding domainMore data for this Ligand-Target Pair
Affinity DataIC50: 2.15E+4nMAssay Description:The 3CLpro enzyme assay was developed in 384-well black, medium binding microplates (Greiner Bio-One, Monroe, NC, USA) with a total volume of 20 _...More data for this Ligand-Target Pair
Affinity DataIC50: 2.15E+4nMAssay Description:This is a review article. Please point to the original journal.More data for this Ligand-Target Pair
Affinity DataIC50: 100nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
Affinity DataIC50: 12nM EC50: 410nMpH: 7.4 T: 2°CAssay Description:Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)