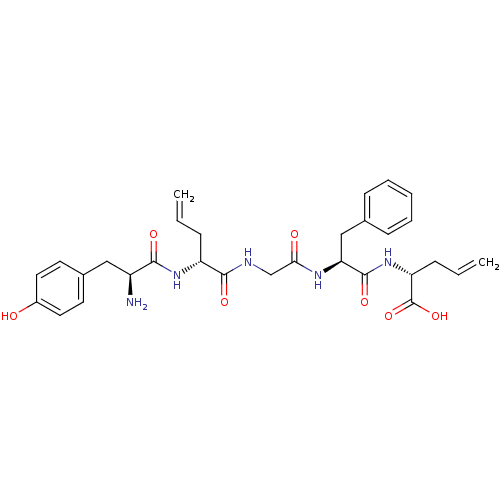
BDBM21128 (2R)-2-[(2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl)propanamido]pent-4-enamido]acetamido}-3-phenylpropanamido]pent-4-enoic acid::[2-D-allylglycine, 5-D-allylglycine]-enkephalin::lDADAE (13)::linear H-Tyr-D-AllylGly-Gly-Phe-D-Allylgly-OH
SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@H](CC=C)C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@H](CC=C)C(O)=O
InChI Key InChIKey=PRTORGAXMSTSIY-LIONHTAISA-N
Data 3 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 21128
Found 3 hits for monomerid = 21128
Affinity DataKi: 8.10nM ΔG°: -11.0kcal/molepH: 7.4 T: 2°CAssay Description:Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe...More data for this Ligand-Target Pair
Affinity DataKi: 64.5nM ΔG°: -9.80kcal/molepH: 7.4 T: 2°CAssay Description:Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe...More data for this Ligand-Target Pair
Affinity DataKi: 3.87E+3nM ΔG°: -7.38kcal/molepH: 7.4 T: 2°CAssay Description:Log IC50 values for each test compound were determined from nonlinear regression analysis of data collected from two independent experiments performe...More data for this Ligand-Target Pair