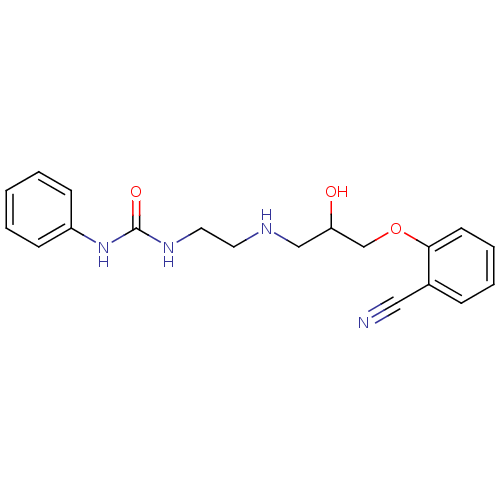
BDBM25748 3-(2-{[3-(2-cyanophenoxy)-2-hydroxypropyl]amino}ethyl)-1-phenylurea::ICI 89,406::ICI 89406
SMILES OC(CNCCNC(=O)Nc1ccccc1)COc1ccccc1C#N
InChI Key InChIKey=HTLWRKRZKFAAAH-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 25748
Found 3 hits for monomerid = 25748
Affinity DataEC50: 1.00E+4nMAssay Description:Agonist activity at human beta3 adrenoceptor expressed in CHOK1 cells assessed as induction of [3H]cAMP accumulation after 5 hrsMore data for this Ligand-Target Pair
TargetBeta-2 adrenergic receptor(Homo sapiens (Human))
Ohio State University
Curated by PDSP Ki Database
Ohio State University
Curated by PDSP Ki Database
Affinity DataEC50: 60nMAssay Description:Agonist activity at human beta2 adrenoceptor expressed in CHOK1 cells assessed as induction of [3H]cAMP accumulation after 5 hrsMore data for this Ligand-Target Pair
Affinity DataEC50: 0.813nMAssay Description:Partial agonist activity at human beta1 adrenoceptor expressed in CHOK1 cells assessed as induction of [3H]cAMP accumulation after 5 hrsMore data for this Ligand-Target Pair