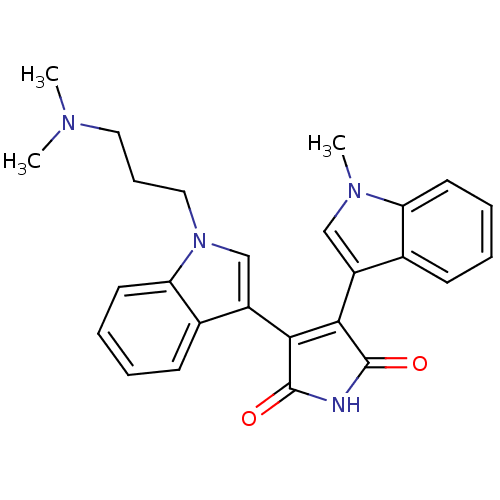
BDBM2681 3-{1-[3-(dimethylamino)propyl]-1H-indol-3-yl}-4-(1-methyl-1H-indol-3-yl)-2,5-dihydro-1H-pyrrole-2,5-dione::Bisindolyl deriv. 11::CHEMBL268769
SMILES CN(C)CCCn1cc(C2=C(C(=O)NC2=O)c2cn(C)c3ccccc23)c2ccccc12
InChI Key InChIKey=WHOOZDLAJIKMBZ-UHFFFAOYSA-N
Data 9 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 2681
Found 9 hits for monomerid = 2681
Affinity DataIC50: 40nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
TargetcAMP-dependent protein kinase catalytic subunit alpha(Rattus norvegicus (rat))
Laboratoires Glaxo
Laboratoires Glaxo
Affinity DataIC50: 3.50E+3nMpH: 7.0 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
TargetPhosphorylase b kinase regulatory subunit alpha, skeletal muscle isoform(Oryctolagus cuniculus (rabbit))
Laboratoires Glaxo
Laboratoires Glaxo
Affinity DataIC50: 1.70E+3nMpH: 7.0 T: 2°CAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 45nMAssay Description:The activity of PKC, activated by phosphatidylerine and Ca2+, is measured by its ability to transfer phosphate from [gamma-32P]ATP to lysine-rich his...More data for this Ligand-Target Pair
Affinity DataIC50: 150nMAssay Description:Inhibition of human recombinant Pim1 expressed in insect cells by HTRFMore data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Inhibition of Protein kinase C alphaMore data for this Ligand-Target Pair
Affinity DataIC50: >4.00E+5nMAssay Description:Inhibitory activity against malate dehydrogenase (MDH) from Thermus flavusMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+5nMAssay Description:Inhibitory activity against Chymotrypsinogen from Thermus flavusMore data for this Ligand-Target Pair
Affinity DataIC50: 2.56E+4nMAssay Description:The activity of PKA, activated by cAMP, is measured by its ability to transfer phosphate from [gamma-32P]ATP to histone.More data for this Ligand-Target Pair