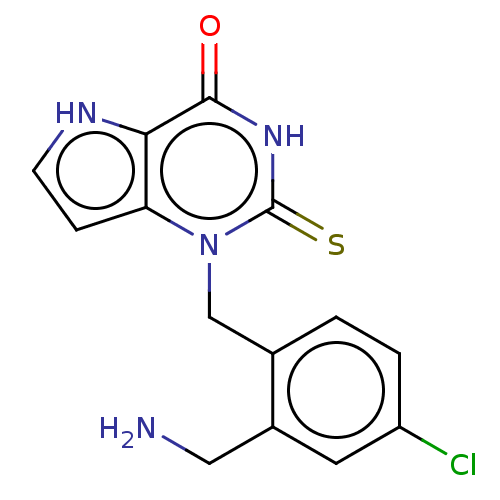
BDBM312244 1-[2-(Aminomethyl)-4-chlorobenzyl]-2-thioxo-1,2,3,5-tetrahydro-4H-pyrrolo[3,2-d]pyrimidin-4-one::US10016430, Example 7::US11000525, Example 7::US9616063, 7
SMILES NCc1cc(Cl)ccc1Cn1c2cc[nH]c2c(=O)[nH]c1=S
InChI Key InChIKey=MFCVOLWLNXXQFY-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 17 hits for monomerid = 312244
Found 17 hits for monomerid = 312244
Affinity DataIC50: 15nMAssay Description:The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the pres...More data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+4nMAssay Description:Competitive inhibition of human recombinant CYP3A4 using coumarin as substrate preincubated with enzyme for 10 mins followed by NADPH addition and me...More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:MPO: The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+4nMAssay Description:MPO: The pharmacological activity of compounds disclosed herein was tested in the following screen (Test A) in which the compounds were tested in the...More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Methods for the determination of MPO inhibitory activity are disclosed in WO 02/090575. The pharmacological activity of compounds disclosed herein wa...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+4nMAssay Description:Methods for the determination of MPO inhibitory activity are disclosed in WO 02/090575. The pharmacological activity of compounds disclosed herein wa...More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Inhibition of MPO (unknown origin) measured after 15 mins in presence of H2O2 by chemiluminescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of MPO (unknown origin) measured after 15 mins in presence of H2O2 by chemiluminescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibition of human recombinant CYP2C9 using coumarin as substrate preincubated with enzyme for 10 mins followed by NADPH addition and measured after...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+4nMAssay Description:Inhibition of MPO in human HL-60 cells measured after 15 mins in presence of H2O2 by chemiluminescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibition of human recombinant TPO (1 to 839 residues) expressed in baculovirus-infected insect cells measured after 15 mins in presence of H2O2 by ...More data for this Ligand-Target Pair
Affinity DataKd: 3.98E+3nMAssay Description:Displacement of benzhydroxamic acid from native state MPO (unknown origin) by 1D NMR reporter assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataIC50: >4.00E+4nMAssay Description:Inhibition of hERGMore data for this Ligand-Target Pair
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin) using coumarin as substrate preincubated with enzyme for 10 mins followed by NADPH addition and measured after ...More data for this Ligand-Target Pair
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibition of human recombinant CYP1A2 using coumarin as substrate preincubated with enzyme for 10 mins followed by NADPH addition and measured after...More data for this Ligand-Target Pair
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibition of human recombinant CYP2C19 using coumarin as substrate preincubated with enzyme for 10 mins followed by NADPH addition and measured afte...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+4nMAssay Description:To detect thyroid peroxidase (TPO) inhibitory activity, the production of hypoiodous acid (HOI) was quantified. HOI was detected by reacting it with ...More data for this Ligand-Target Pair