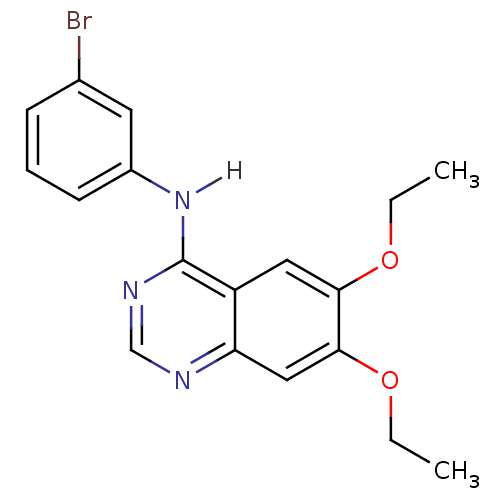
BDBM3556 4-[(3-Bromophenyl)amino]-6,7-diethoxyquinazoline::CHEMBL1204199::CHEMBL35820::N-(3-bromophenyl)-6,7-diethoxyquinazolin-4-amine::PD153035 Analog::PD153035 Analog 56
SMILES CCOc1cc2ncnc(Nc3cccc(Br)c3)c2cc1OCC
InChI Key InChIKey=YXOXHAUUTIOBDA-UHFFFAOYSA-N
Data 10 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 3556
Found 10 hits for monomerid = 3556
Affinity DataIC50: 0.00600nMAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 0.00603nMAssay Description:Inhibition of EGFRMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Compound was evaluated for its concentration required to inhibit the porcine kidney F16BPaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMAssay Description:Concentration required to inhibit the human liver recombinant fructose-1,6-bisphosphatase.More data for this Ligand-Target Pair
Affinity DataIC50: 0.00600nMAssay Description:Inhibition of wild type EGFRChecked by AuthorMore data for this Ligand-Target Pair
TargetReceptor tyrosine-protein kinase erbB-2(Homo sapiens (Human))
Lawrence Berkeley National Laboratory
Curated by ChEMBL
Lawrence Berkeley National Laboratory
Curated by ChEMBL
Affinity DataIC50: 215nMAssay Description:Inhibition of human ErbB2 tyrosine kinase phosphorylation expressed in mouse BaF3 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 0.410nMAssay Description:Displacement of [125I]4-(3-iodoanilino)-6,7-dimethoxyquinazoline from EGFR tyrosine kinase in human A431 cell membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:Inhibition of human EGFR tyrosine kinase phosphorylation expressed in mouse BaF3 cellsMore data for this Ligand-Target Pair
TargetReceptor tyrosine-protein kinase erbB-4(Homo sapiens (Human))
Lawrence Berkeley National Laboratory
Curated by ChEMBL
Lawrence Berkeley National Laboratory
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Inhibition of human ErbB4 tyrosine kinase phosphorylation expressed in human CEM/4 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Compound was evaluated for its concentration required to inhibit the rat liver F16BPaseMore data for this Ligand-Target Pair