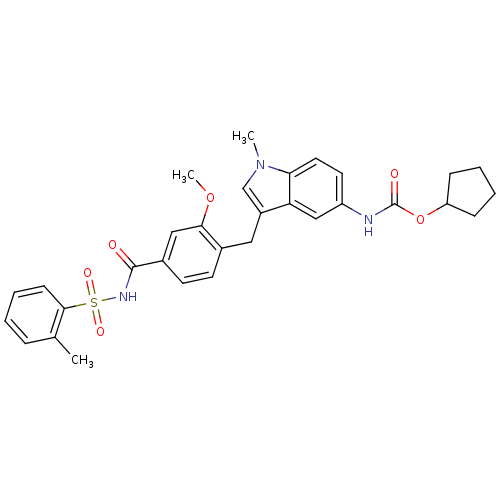
BDBM50009073 4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol-3-ylmethyl)-3-methoxy-N-o-tolylsulfonylbenzamide::CHEMBL603::ZAFIRLUKAST::cyclopentyl 3-(2-methoxy-4-((o-tolylsulfonyl)carbamoyl)benzyl)-1-methylindole-5-carbamate::cyclopentyl 3-[2-methoxy-4-(2-methylphenylsulfonylcarbamoyl)benzyl]-1-methyl-1H-indol-5-ylcarbamate
SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1C
InChI Key InChIKey=YEEZWCHGZNKEEK-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50009073
Found 3 hits for monomerid = 50009073
Affinity DataKi: 0.300nMAssay Description:Displacement of [3H]LTD4 from cysteinyl leukotriene receptor 1 in Hartley guinea pig parenchymal membrane after 30 mins by liquid scintillation count...More data for this Ligand-Target Pair
TargetCysteinyl leukotriene receptor 1(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Inhibition of cysteinyl leukotriene receptor 1 (unknown origin) expressed in HEK293 cell membranes after 45 mins by scintillation spectrometryMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Homo sapiens (Human))
Goethe-University Frankfurt
Curated by ChEMBL
Goethe-University Frankfurt
Curated by ChEMBL
Affinity DataEC50: 3.06E+4nMAssay Description:Activation of human PXR expressed in DPX2 cells assessed as CYP3A4 induction after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)