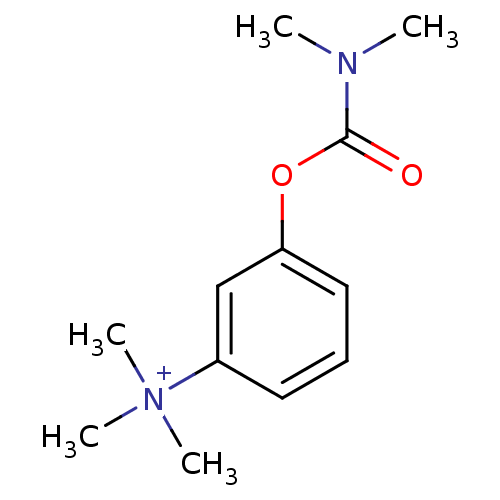
BDBM50022775 (m-Hydroxyphenyl)trimethylammonium dimethylcarbamate::3-Trimethylammoniumphenyl N,N-dimethylcarbamate::3-[(dimethylcarbamoyl)oxy]-N,N,N-trimethylanilinium::CHEMBL211471::CHEMBL278020::Eustigmin::Eustigmine::NEOSTIGMINE::Neostigmine (2)::Prostigmine::Vagostigmine::m-Trimethylammoniumphenyldimethylcarbamate
SMILES CN(C)C(=O)Oc1cccc(c1)[N+](C)(C)C
InChI Key InChIKey=ALWKGYPQUAPLQC-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 12 hits for monomerid = 50022775
Found 12 hits for monomerid = 50022775
Affinity DataKi: 4.80E+4nMAssay Description:Inhibition assay using AChE and BuChE.More data for this Ligand-Target Pair
Affinity DataIC50: 800nMpH: 7.4Assay Description:Human erythrocyte AChE or human plasmatic BChE (Prague, Aldrich; commercially purified by affinity chromatography) were suspended into phosphate buff...More data for this Ligand-Target Pair
Affinity DataIC50: 4.91E+4nMAssay Description:Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins before substrate addition after 15 mins by Ellman's me...More data for this Ligand-Target Pair
Affinity DataIC50: 4.96E+4nMAssay Description:Inhibition of BuChE in equine serum using butyrylthiocholine chloride as substrate preincubated with enzyme for 10 mins prior to substrate challenge ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.61E+4nMAssay Description:Inhibition of human BChE using butyrylthiocholine iodide as substrate preincubated for 10 mins before substrate addition after 15 mins by Ellman's me...More data for this Ligand-Target Pair
Affinity DataIC50: 1.63E+4nMAssay Description:Inhibition of human BChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 70nMAssay Description:Inhibition of equine serum BChE using butylthiocholine iodide as substrate after 10 mins by Ellman methodMore data for this Ligand-Target Pair
Affinity DataIC50: 60nMAssay Description:Inhibition of human plasma BChE pretreated for 30 mins by Ellman techniqueMore data for this Ligand-Target Pair
Affinity DataIC50: 2.93E+3nMAssay Description:Inhibition of equine BuChE by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 9.27E+4nMAssay Description:Inhibition of horse serum BChE incubated for 5 mins by DTNB reagent based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 160nMAssay Description:Inhibition of equine BChE using acetylthiocholine chloride as substrate preincubated for 15 mins followed by substrate addition and measured after 30...More data for this Ligand-Target Pair
Affinity DataIC50: 800nMAssay Description:Inhibition of human plasma BChE after 5 mins by Ellman's methodMore data for this Ligand-Target Pair