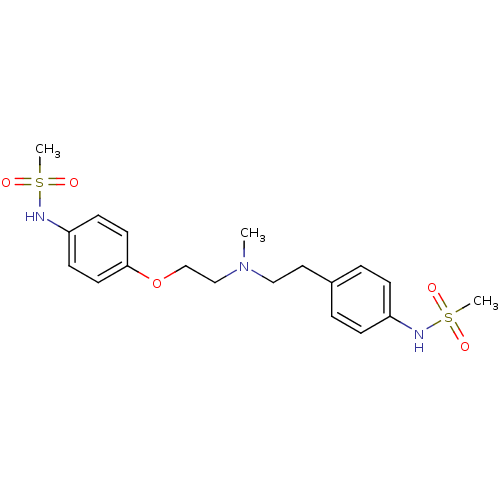
BDBM50031720 (Dofetilide) N-[4-(2-{[2-(4-Methanesulfonylamino-phenyl)-ethyl]-methylamino}-ethoxy)-phenyl]-methanesulfonamide::CHEMBL473::DOFETILIDE::N-[4-(2-{[2-(4-METHANESULFONYLAMINO-PHENYL)-ETHYL]-METHYL-AMINO}-ETHOXY)-PHENYL]-METHANESULFONAMIDE DOFETILIDE::N-[4-(2-{[2-(4-Methanesulfonylamino-phenyl)-ethyl]-methyl-amino}-ethoxy)-phenyl]-methanesulfonamide (UK-68798)::N-[4-(2-{[2-(4-Methanesulfonylamino-phenyl)-ethyl]-methyl-amino}-ethoxy)-phenyl]-methanesulfonamide (dofetilide)::N-[4-(2-{[2-(4-methanesulfonylamino-phenoxy)-ethyl]-methyl-amino}-ethyl)-phenyl]-methanesulfonamide::TIKOSYN::UK-68,798::US10167299, Dofetilide
SMILES CN(CCOc1ccc(NS(C)(=O)=O)cc1)CCc1ccc(NS(C)(=O)=O)cc1
InChI Key InChIKey=IXTMWRCNAAVVAI-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 1 hit for monomerid = 50031720
Found 1 hit for monomerid = 50031720
Shanghai Institute of Material Medica, Chinese Academy of Sciences
US Patent