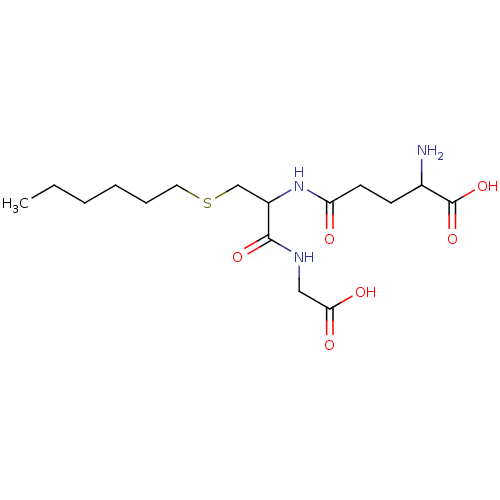
BDBM50043758 2-Amino-4-[1-(carboxymethyl-carbamoyl)-2-hexylsulfanyl-ethylcarbamoyl]-butyric acid::CHEMBL58135
SMILES CCCCCCSCC(NC(=O)CCC(N)C(O)=O)C(=O)NCC(O)=O
InChI Key InChIKey=HXJDWCWJDCOHDG-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50043758
Found 4 hits for monomerid = 50043758
Affinity DataKi: 840nMAssay Description:Inhibitory activity of the compound was measured on recombinant human Glutathione-S-transferase A1 enzymeMore data for this Ligand-Target Pair
Affinity DataKi: 2.00E+3nMAssay Description:Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase Mu 1More data for this Ligand-Target Pair
Affinity DataKi: 1.00E+4nMAssay Description:Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase PMore data for this Ligand-Target Pair
Affinity DataKi: 3.60E+4nMAssay Description:Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase Mu 2More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)