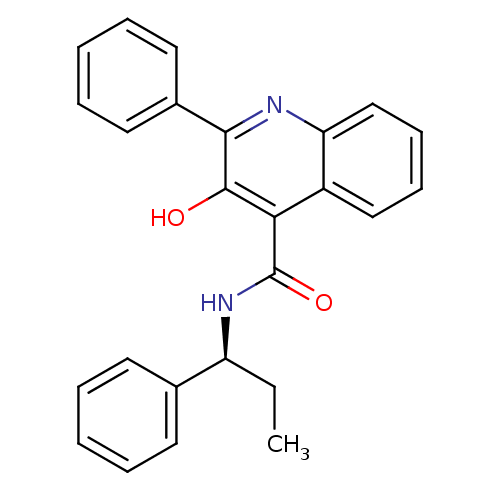
BDBM50051293 (S)-(-)-N-(R-ethylbenzyl)-3-hydroxy-2-phenylquinoline-4-carboxamide::3-Hydroxy-2-phenyl-quinoline-4-carboxylic acid ((S)-1-phenyl-propyl)-amide::3-Hydroxy-2-phenyl-quinoline-4-carboxylic acid (1-phenyl-propyl)-amide::CHEMBL10188::SB 223412::SB-2234::SB-223412::Talnetant
SMILES CC[C@H](NC(=O)c1c(O)c(nc2ccccc12)-c1ccccc1)c1ccccc1
InChI Key InChIKey=BIAVGWDGIJKWRM-FQEVSTJZSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50051293
Found 3 hits for monomerid = 50051293
TargetNeuromedin-K receptor(Homo sapiens (Human))
Northeastern Ohio Universities Colleges Of Medicine And Pharmacy
Curated by ChEMBL
Northeastern Ohio Universities Colleges Of Medicine And Pharmacy
Curated by ChEMBL
Affinity DataKi: 2.51nMAssay Description:Displacement of [125I]-MePhe7-NKB from human NK3 receptor expressed in CHO cells after 90 mins by gamma countingMore data for this Ligand-Target Pair
TargetNeuromedin-K receptor(Homo sapiens (Human))
Northeastern Ohio Universities Colleges Of Medicine And Pharmacy
Curated by ChEMBL
Northeastern Ohio Universities Colleges Of Medicine And Pharmacy
Curated by ChEMBL
Affinity DataIC50: 6.10nMAssay Description:Antagonist activity against human NK3 receptor expressed in HEK293 cells assessed as inhibition of increase of intracellular calcium level by fluores...More data for this Ligand-Target Pair
TargetNeuromedin-K receptor(Homo sapiens (Human))
Northeastern Ohio Universities Colleges Of Medicine And Pharmacy
Curated by ChEMBL
Northeastern Ohio Universities Colleges Of Medicine And Pharmacy
Curated by ChEMBL
Affinity DataIC50: 16.6nMAssay Description:Antagonist activity against human NK3 receptor expressed in HEK293 cells assessed as inhibition of NKB-induced increase of intracellular calcium leve...More data for this Ligand-Target Pair