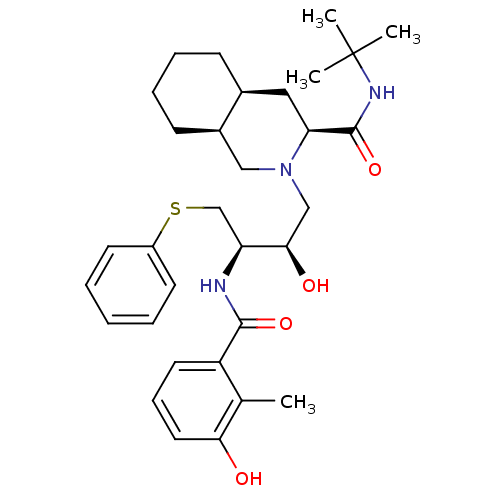
BDBM50061306 (3S,4aS,8aS)-2-[(2R,3R)-2-Hydroxy-3-(3-hydroxy-2-methyl-benzoylamino)-4-phenylsulfanyl-butyl]-decahydro-isoquinoline-3-carboxylic acid tert-butylamide; compound with methanesulfonic acid::AG-1343::CHEMBL1205::NELFINAVIR MESYLATE::Nelfinavir::Viracept::cmdc.202100576, 24h
SMILES Cc1c(O)cccc1C(=O)N[C@@H](CSc1ccccc1)[C@H](O)CN1C[C@H]2CCCC[C@H]2C[C@H]1C(=O)NC(C)(C)C
InChI Key InChIKey=QAGYKUNXZHXKMR-HKWSIXNMSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 11 hits for monomerid = 50061306
Found 11 hits for monomerid = 50061306
TargetProtease(Human immunodeficiency virus 1 (HIV-1))
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Affinity DataKi: 0.0700nMAssay Description:Inhibition of wild-type HIV1 BH10 protease expressed in Escherichia coli by spectrophotometric assayMore data for this Ligand-Target Pair
TargetProtease(Human immunodeficiency virus 1 (HIV-1))
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Affinity DataKi: 0.931nMAssay Description:Inhibition of HIV1 proteaseMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Binding affinity to HIV1 protease assessed as inhibition constantMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Inhibitory activity against HIV-1 proteaseMore data for this Ligand-Target Pair
TargetHIV-1 protease(Human immunodeficiency virus)
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Affinity DataKi: 3.20nMAssay Description:Inhibition of HIV1 recombinant protease A71V/V82T/I84V mutant expressed in Escherichia coli by spectrophotometric assayMore data for this Ligand-Target Pair
TargetHIV-1 protease(Human immunodeficiency virus)
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Affinity DataKi: 3.80nMAssay Description:Inhibition of HIV1 recombinant protease M46I/A71V/V82T/I84V mutant expressed in Escherichia coli by spectrophotometric assayMore data for this Ligand-Target Pair
TargetHIV-1 protease(Human immunodeficiency virus)
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:Inhibition of HIV1 recombinant protease V32I/I47A mutant expressed in Escherichia coli by spectrophotometric assayMore data for this Ligand-Target Pair
TargetHIV-1 protease(Human immunodeficiency virus)
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Affinity DataKi: 18nMAssay Description:Inhibition of HIV1 recombinant protease D30N/N88D mutant expressed in Escherichia coli by spectrophotometric assayMore data for this Ligand-Target Pair
TargetHIV-1 protease(Human immunodeficiency virus)
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Academy Of Sciences Of The Czech Republic
Curated by ChEMBL
Affinity DataKi: 32nMAssay Description:Inhibition of HIV1 recombinant protease L10F/L19I/K20R/L33F/E35D/M36I/R41K/F53L/I54V/L63P/H69K/A71V/T74P/I84V/L89M/L90M/I93L mutant expressed in Esch...More data for this Ligand-Target Pair
Affinity DataKi: 480nMAssay Description:Mechanism based inhibition of human cytochrome P450 3A4 measured by testosterone hydroxylationMore data for this Ligand-Target Pair
Affinity DataKi: 570nMAssay Description:Mechanism based inhibition of human cytochrome P450 3A5 measured by testosterone hydroxylationMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)