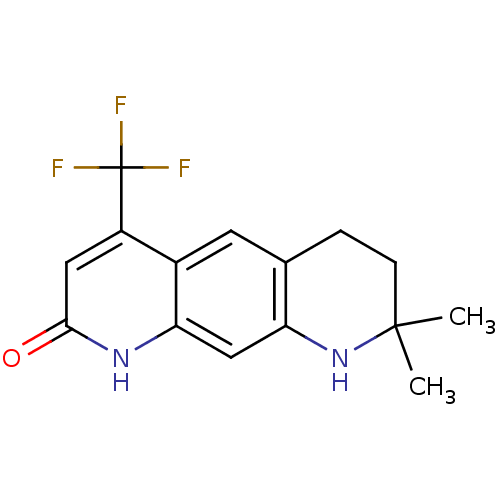BDBM50063127 8,8-Dimethyl-4-trifluoromethyl-6,7,8,9-tetrahydro-1H-pyrido[3,2-g]quinolin-2-one::CHEMBL6874
SMILES CC1(C)CCc2cc3c(cc(=O)[nH]c3cc2N1)C(F)(F)F
InChI Key InChIKey=LCTXHPZYTFNYEN-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 50063127
Found 10 hits for monomerid = 50063127
Affinity DataKi: 26nMAssay Description:Inhibition of human androgen receptorMore data for this Ligand-Target Pair
Affinity DataKi: 26nMAssay Description:Binding affinity for human androgen receptor in transiently-transfected COS-1 cells.More data for this Ligand-Target Pair
Affinity DataKi: 26nMAssay Description:Binding affinity towards human androgen receptor (hAR), using dihydrotestosterone as radioligand for competitive binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 26nMAssay Description:Binding affinity for human androgen receptor transfected into mammalian COS-1 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:Inhibition of human androgen receptorMore data for this Ligand-Target Pair
Affinity DataEC50: <1.00E+4nMAssay Description:Agonistic activity against human androgen receptor (hAR) expressed in CV-1 cell linesMore data for this Ligand-Target Pair
Affinity DataIC50: 330nMAssay Description:Antagonistic activity against human progesterone receptor B (hPR-B) in co-transfected CV-1 cells.More data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:Inhibitory concentration against human androgen receptor (AR) dependent transcriptional activity in cotransfected mammalian CV-1 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:Antagonistic activity against human androgen receptor (hAR) expressed in CV-1 cell linesMore data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:Antagonistic activity against human androgen receptor (hAR) in co-transfected CV-1 cells.More data for this Ligand-Target Pair