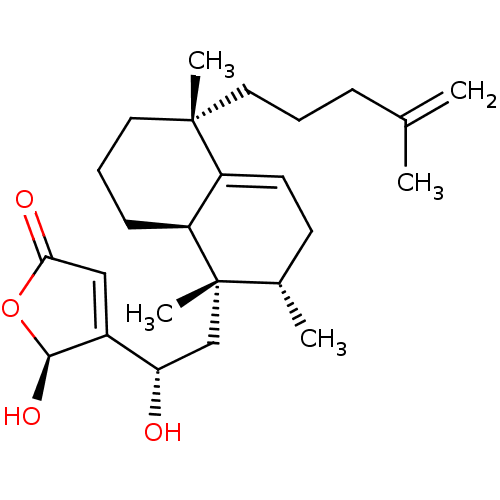
BDBM50068035 (S)-5-Hydroxy-4-{(S)-1-hydroxy-2-[(1S,2S,5R,8aR)-1,2,5-trimethyl-5-(4-methyl-pent-4-enyl)-1,2,3,5,6,7,8,8a-octahydro-naphthalen-1-yl]-ethyl}-5H-furan-2-one::5-Hydroxy-4-{1-hydroxy-2-[1,2,5-trimethyl-5-(4-methyl-pent-4-enyl)-1,2,3,5,6,7,8,8a-octahydro-naphthalen-1-yl]-ethyl}-5H-furan-2-one(dysidiolide)::CHEMBL356927
SMILES C[C@H]1CC=C2[C@H](CCC[C@]2(C)CCCC(C)=C)[C@@]1(C)C[C@H](O)C1=CC(=O)O[C@@H]1O
InChI Key InChIKey=JOYRNJIWPUZFBJ-LAXAASGQSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50068035
Found 2 hits for monomerid = 50068035
Affinity DataIC50: 1.50E+4nMAssay Description:The compound was tested for its inhibitory activity against Cell division cycle 25AMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+4nMAssay Description:The compound was tested for its inhibitory activity against Cell division cycle 25BMore data for this Ligand-Target Pair