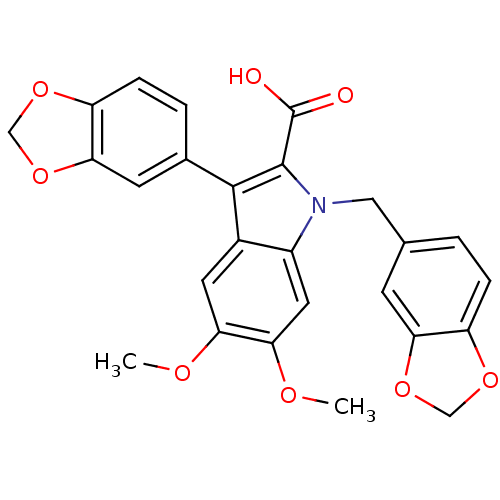
BDBM50069565 3-Benzo[1,3]dioxol-5-yl-1-benzo[1,3]dioxol-5-ylmethyl-5,6-dimethoxy-1H-indole-2-carboxylic acid::CHEMBL276638
SMILES COc1cc2c(c(C(O)=O)n(Cc3ccc4OCOc4c3)c2cc1OC)-c1ccc2OCOc2c1
InChI Key InChIKey=QXUMYHYMXFAQPE-UHFFFAOYSA-N
Data 5 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50069565
Found 5 hits for monomerid = 50069565
Affinity DataIC50: 150nMAssay Description:Inhibition of [125I]-ET-1 binding to rat aorta membrane Endothelin A receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of [125I]-ET-1 binding to porcine kidney inner medulla membrane Endothelin B receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 60nMAssay Description:Inhibitory concentration to the endothelin A receptor expressed in LtK cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibitory concentration against Endothelin B receptor expressed in CHO-K1 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 150nMAssay Description:Potency of the compound at Endothelin A receptor was determinedMore data for this Ligand-Target Pair