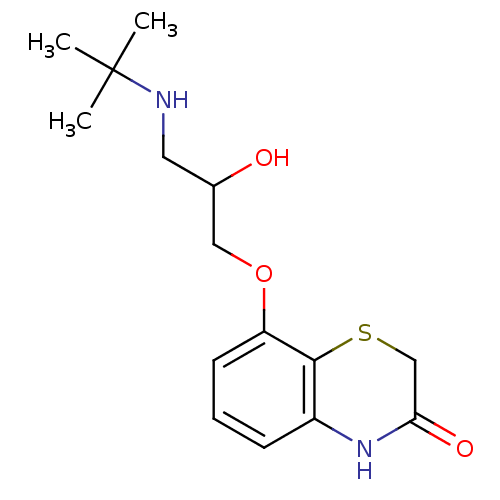
BDBM50086673 8-(3-tert-Butylamino-2-hydroxy-propoxy)-4H-benzo[1,4]thiazin-3-one::CHEMBL140908
SMILES CC(C)(C)NCC(O)COc1cccc2NC(=O)CSc12
InChI Key InChIKey=SWWQDUZAPYPHEI-UHFFFAOYSA-N
Data 4 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50086673
Found 4 hits for monomerid = 50086673
Affinity DataKi: 20nMAssay Description:Compound was evaluated for its Beta-1 adrenergic receptor binding affinity by measuring the displacement of [3H]-dihydroalprenolol binding in rat hea...More data for this Ligand-Target Pair
Affinity DataKi: 23nMAssay Description:Beta-2 adrenergic receptor binding affinity by measuring the displacement of [3H]-DHA binding in rat lungMore data for this Ligand-Target Pair
TargetAlpha-1A/Alpha-1B/Alpha-1D adrenergic receptor(Rattus norvegicus (rat))
Università
Curated by ChEMBL
Università
Curated by ChEMBL
Affinity DataKi: >1.00E+3nMAssay Description:Alpha-1 adrenergic receptor binding affinity by measuring the displacement of [3H]-prazosin binding in rat brain; Not Active means Ki >1000 nMMore data for this Ligand-Target Pair
TargetAlpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor(RAT)
Università
Curated by ChEMBL
Università
Curated by ChEMBL
Affinity DataKi: >1.00E+3nMAssay Description:Alpha-2 adrenergic receptor binding affinity by measuring the displacement of [3H]clonidine binding in rat cerebral cortex; Not Active means Ki >1000...More data for this Ligand-Target Pair