BDBM50088494 BDBM50101974::CHEMBL3542292::CI-991::CS-045::GR-92132X::Gr92132X::Prelay::Rezulin::TROGLITAZONE
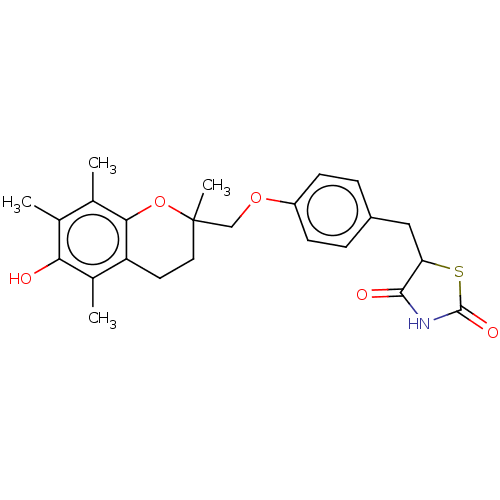
SMILES Cc1c(c2c(c(c1O)C)CC[C@](O2)(C)COc3ccc(cc3)C[C@@H]4C(=O)NC(=O)S4)C
InChI Key InChIKey=GXPHKUHSUJUWKP-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50088494
Found 9 hits for monomerid = 50088494
TargetPeroxisome proliferator-activated receptor gamma(Human)
Glaxo Wellcome Research and Development
Curated by ChEMBL
Glaxo Wellcome Research and Development
Curated by ChEMBL
Affinity DataKi: 302nMAssay Description:Binding affinity against Peroxisome proliferator activated receptor gamma (PPAR gamma)More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Human)
Glaxo Wellcome Research and Development
Curated by ChEMBL
Glaxo Wellcome Research and Development
Curated by ChEMBL
Affinity DataEC50: 537nMAssay Description:Transcriptional activation of peroxisome proliferator activated receptor gammaMore data for this Ligand-Target Pair
Affinity DataIC50: 2.70E+3nMAssay Description:Inhibition of human BSEP expressed in plasma membrane vesicles of Sf21 cells assessed as inhibition of ATP-dependent [3H]taurocholate uptakeMore data for this Ligand-Target Pair
Affinity DataIC50: 5.90E+3nMAssay Description:Inhibition of human BSEP expressed in fall armyworm sf9 cell plasma membrane vesicles assessed as reduction in vesicle-associated [3H]-taurocholate t...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Human)
National Institutes of Health Chemical Genomics Center
Curated by ChEMBL
National Institutes of Health Chemical Genomics Center
Curated by ChEMBL
Affinity DataEC50: 6.90E+3nMAssay Description:Activation of human PXR expressed in human HepG2 (DPX-2) cells assessed as induction of CYP3A4 after 24 hrs by luminescent analysisMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Human)
National Institutes of Health Chemical Genomics Center
Curated by ChEMBL
National Institutes of Health Chemical Genomics Center
Curated by ChEMBL
Affinity DataEC50: 8.90E+3nMAssay Description:Competitive binding affinity to human PXR LBD (111 to 434) by TR-FRET assayMore data for this Ligand-Target Pair
TargetVoltage-dependent L-type calcium channel subunit alpha-1C(Rat)
Jagiellonian University
Curated by ChEMBL
Jagiellonian University
Curated by ChEMBL
Affinity DataIC50: 9.50E+3nMAssay Description:Inhibition of L-type calcium channel measured using whole-cell patch clamp in rat ventricular myocytesMore data for this Ligand-Target Pair
Affinity DataIC50: 1.06E+4nMAssay Description:Inhibition of Sprague-Dawley rat Bsep expressed in plasma membrane vesicles of Sf21 cells assessed as inhibition of ATP-dependent [3H]taurocholate up...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Human)
National Institutes of Health Chemical Genomics Center
Curated by ChEMBL
National Institutes of Health Chemical Genomics Center
Curated by ChEMBL
Affinity DataEC50: 3.16E+4nMAssay Description:Activation of human PXR expressed in human HepG2 (DPX-2) cells after 24 hrs by luciferase reporter gene based luminescent analysisMore data for this Ligand-Target Pair