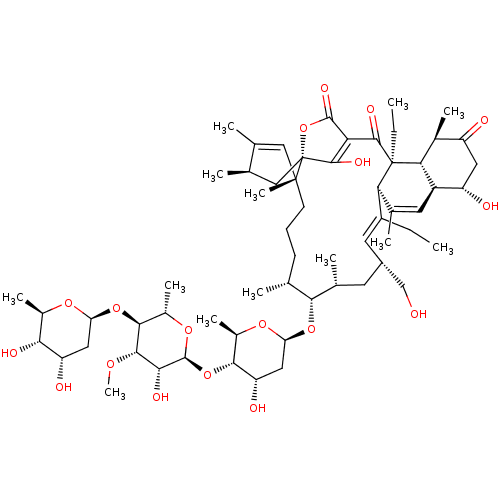
BDBM50088528 CHEMBL3576921
SMILES [H][C@@]1(C[C@H](O)[C@H](O)[C@@H](C)O1)O[C@H]1[C@H](C)O[C@@]([H])(O[C@H]2[C@@H](O)C[C@]([H])(O[C@@H]3[C@H](C)CCC[C@]4(C)C=C(C)[C@H](C)C[C@]44OC(=O)C(=C4O)C(=O)[C@@]4(CC)[C@]5([H])[C@@H](C)C(=O)C[C@H](O)[C@@]5([H])C=C(C)[C@@]4([H])\C(CC)=C/[C@H](CO)C[C@H]3C)O[C@@H]2C)[C@H](O)[C@@H]1OC
InChI Key InChIKey=DRKBZEJUOBNVRH-TXWYELBGSA-N
Data 1 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 1 hit for monomerid = 50088528
Found 1 hit for monomerid = 50088528
TargetEndoplasmic reticulum chaperone BiP(Homo sapiens (Human))
University Of California San Diego
Curated by ChEMBL
University Of California San Diego
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:Inhibition of 2-deoxyglucose-induced GRP78 (unknown origin) expression transfected in human HT1080 cells by luciferase reporter gene assayMore data for this Ligand-Target Pair