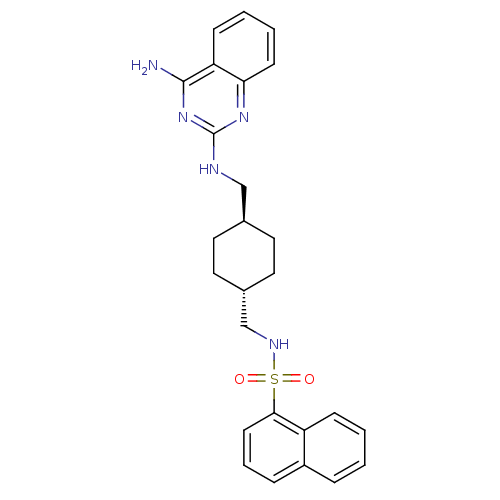
BDBM50089038 CGP 71683::CGP-71683A::CHEMBL17645::N-{[(1r,4r)-4-{[(4-aminoquinazolin-2-yl)amino]methyl}cyclohexyl]methyl}naphthalene-1-sulfonamide (Compound 1)::Naphthalene-1-sulfonic acid {4-[(4-amino-quinazolin-2-ylamino)-methyl]-cyclohexylmethyl}-amide
SMILES Nc1nc(NC[C@H]2CC[C@H](CNS(=O)(=O)c3cccc4ccccc34)CC2)nc2ccccc12
InChI Key InChIKey=UULIGRNKXHCLQN-WGSAOQKQSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 50089038
Found 10 hits for monomerid = 50089038
Affinity DataIC50: 2.90nMAssay Description:Human Neuropeptide Y5 receptor binding affinityMore data for this Ligand-Target Pair
Affinity DataIC50: 2.90nMAssay Description:Compound was tested for its antagonistic activity against Neuropeptide Y receptor Y5 subtype stably expressed in LM(tk-)cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 5.74E+3nMAssay Description:Compound was tested for human Neuropeptide Y receptor type 4More data for this Ligand-Target Pair
Affinity DataIC50: 8.37E+3nMAssay Description:Compound was tested for human Neuropeptide Y receptor type 1More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Compound was tested for rat Neuropeptide Y receptor type 5More data for this Ligand-Target Pair
Affinity DataIC50: 2.90nMAssay Description:Compound was tested for human Neuropeptide Y receptor type 5More data for this Ligand-Target Pair
Affinity DataIC50: 2.70E+3nMAssay Description:Osmotic water permeability was measured at 22-24 °C bymonitoring 90° scattered light intensity at 520 nm wavelength. Measurements were made using a P...More data for this Ligand-Target Pair
Affinity DataIC50: 1.89E+3nMAssay Description:Compound was tested for human Neuropeptide Y receptor type 2More data for this Ligand-Target Pair