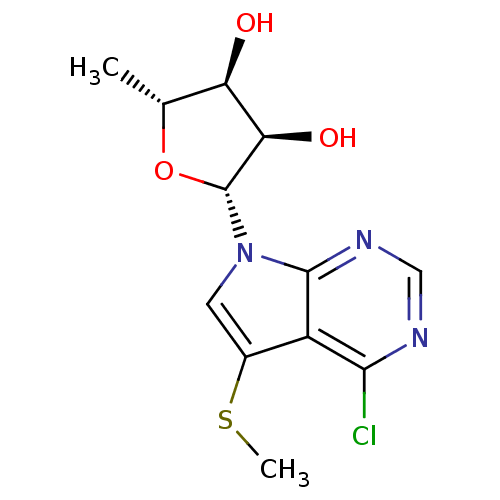
BDBM50090866 (2R,3R,4S,5R)-2-(4-Chloro-5-methylsulfanyl-pyrrolo[2,3-d]pyrimidin-7-yl)-5-methyl-tetrahydro-furan-3,4-diol::(2R,3R,4S,5R)-2-(4-chloro-5-(methylthio)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)-5-methyl-tetrahydrofuran-3,4-diol::2-(4-Chloro-5-methylsulfanyl-pyrrolo[2,3-d]pyrimidin-7-yl)-5-methyl-tetrahydro-furan-3,4-diol::CHEMBL318056
SMILES CSc1cn([C@@H]2O[C@H](C)[C@@H](O)[C@H]2O)c2ncnc(Cl)c12
InChI Key InChIKey=GFGFBPLBDBDSPS-JJNLEZRASA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50090866
Found 4 hits for monomerid = 50090866
Affinity DataIC50: 70nMAssay Description:Inhibition of recombinant human adenosine kinaseMore data for this Ligand-Target Pair
Affinity DataIC50: 7.00E+7nMAssay Description:Inhibition of adenosine kinase (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 70.8nMAssay Description:Concentration required for 50% inhibition of the adenosine kinase (AK) activity.More data for this Ligand-Target Pair
Affinity DataIC50: 70nMAssay Description:Inhibition of human adenosine kinase activityMore data for this Ligand-Target Pair