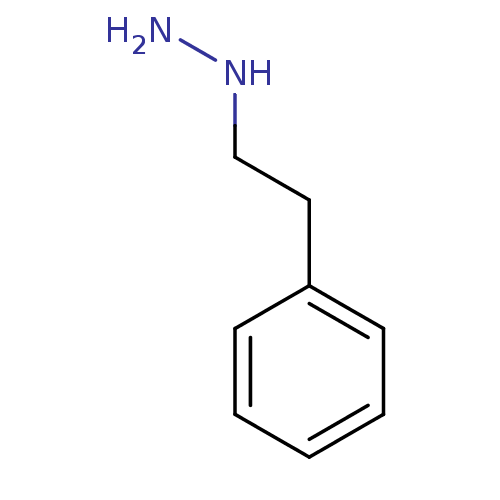
BDBM50105417 CHEMBL1089::Nardil::PHENELZINE::Phenethyl-hydrazine
SMILES NNCCc1ccccc1
InChI Key InChIKey=RMUCZJUITONUFY-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50105417
Found 4 hits for monomerid = 50105417
Affinity DataKi: 124nMAssay Description:Mixed-type inhibition of recombinant human MAO-B assessed as reduction in 4-hydroxyquinoline formation using varying levels of kynuramine as substrat...More data for this Ligand-Target Pair
Affinity DataKi: 163nMAssay Description:Mixed-type inhibition of recombinant human MAO-A assessed as reduction in 4-hydroxyquinoline formation using varying levels of kynuramine as substrat...More data for this Ligand-Target Pair
Affinity DataIC50: 238nMAssay Description:Inhibition of recombinant human MAO-B assessed as reduction in 4-hydroxyquinoline formation using kynuramine as substrate after 20 mins by fluorometr...More data for this Ligand-Target Pair
Affinity DataIC50: 143nMAssay Description:Inhibition of recombinant human MAO-B assessed as reduction in 4-hydroxyquinoline formation using kynuramine as substrate after 20 mins by fluorometr...More data for this Ligand-Target Pair