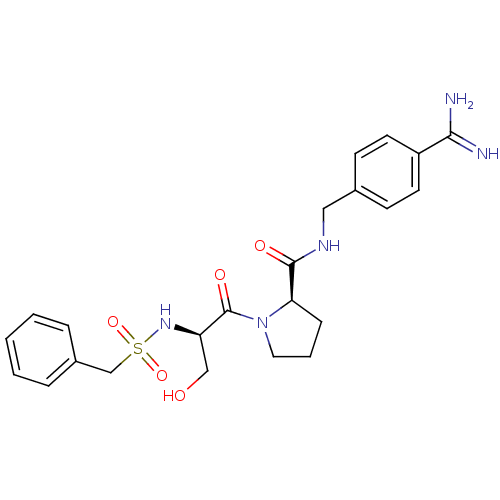
BDBM50110023 2-(3-Hydroxy-2-phenylmethanesulfonylamino-propionyl)-pyrrolidine-1-carboxylic acid 4-carbamimidoyl-benzylamide::CHEMBL158939
SMILES NC(=N)c1ccc(CNC(=O)[C@H]2CCCN2C(=O)[C@@H](CO)NS(=O)(=O)Cc2ccccc2)cc1
InChI Key InChIKey=BCXSNIMTARZGHJ-WOJBJXKFSA-N
Data 5 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50110023
Found 5 hits for monomerid = 50110023
Affinity DataKi: 3.20nMAssay Description:In vitro inhibition of trypsin.More data for this Ligand-Target Pair
Affinity DataKi: 12nMAssay Description:In vitro inhibition of thrombin.More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:In vitro inhibition of plasminogen activator urokinase.More data for this Ligand-Target Pair
Affinity DataKi: 150nMAssay Description:In vitro inhibition of Plasmin.More data for this Ligand-Target Pair
Affinity DataKi: 2.00E+3nMAssay Description:In vitro inhibition of Coagulation factor Xa.More data for this Ligand-Target Pair