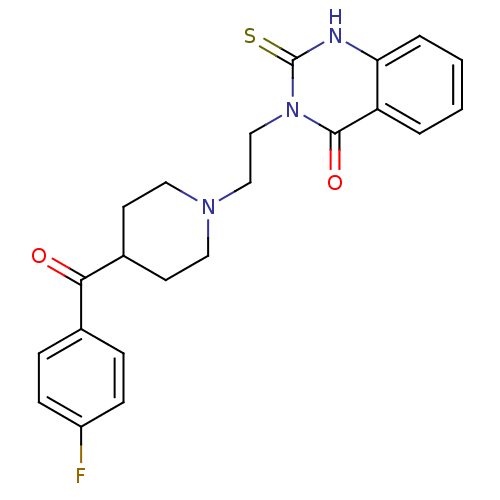
BDBM50113332 3-(2-(4-(4-fluorobenzoyl)piperidin-1-yl)ethyl)-2-thioxo-2,3-dihydroquinazolin-4(1H)-one::3-{2-[4-(4-Fluoro-benzoyl)-piperidin-1-yl]-ethyl}-2-thioxo-2,3-dihydro-1H-quinazolin-4-one::CHEMBL62919::altanserin
SMILES Fc1ccc(cc1)C(=O)C1CCN(CCn2c(=S)[nH]c3ccccc3c2=O)CC1
InChI Key InChIKey=SMYALUSCZJXWHG-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 16 hits for monomerid = 50113332
Found 16 hits for monomerid = 50113332
Target5-hydroxytryptamine receptor 2A(Rattus norvegicus (rat))
Beijing Normal University
Curated by ChEMBL
Beijing Normal University
Curated by ChEMBL
Affinity DataKi: 0.130nMAssay Description:Displacement of [3H]-Ketanserin from rat frontal cortex 5-HT2A receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.130nMAssay Description:Binding affinity to 5HT2A receptorMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2A(Rattus norvegicus (rat))
Beijing Normal University
Curated by ChEMBL
Beijing Normal University
Curated by ChEMBL
Affinity DataKi: 0.130nMAssay Description:Displacement of [3H]altanserine from rat cortical membrane 5HT2A receptorMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Binding affinity against 5-hydroxytryptamine 2A receptor in humansMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2A(Rattus norvegicus (rat))
Beijing Normal University
Curated by ChEMBL
Beijing Normal University
Curated by ChEMBL
Affinity DataKi: 0.720nMAssay Description:Displacement of [3H]MDL from rat 5HT2A receptor expressed in GF62 cells by liquid scintillation analyserMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2A(Rattus norvegicus (rat))
Beijing Normal University
Curated by ChEMBL
Beijing Normal University
Curated by ChEMBL
Affinity DataKi: 0.740nMAssay Description:Displacement of [3H]MDL from rat 5HT2A receptor expressed in GF62 cellsMore data for this Ligand-Target Pair
TargetAlpha-1A adrenergic receptor(Rattus norvegicus (Rat))
Beijing Normal University
Curated by ChEMBL
Beijing Normal University
Curated by ChEMBL
Affinity DataKi: 5nMAssay Description:Displacement of [3H]-WB4101 from rat forebrain alpha 1 adrenergic receptorsMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2C(Homo sapiens (Human))
Institute Of Nuclear Chemistry Johannes Gutenberg-University Mainz
Curated by ChEMBL
Institute Of Nuclear Chemistry Johannes Gutenberg-University Mainz
Curated by ChEMBL
Affinity DataKi: 40nMAssay Description:Binding affinity to 5HT2C receptor (unknown origin) by competitive binding experimentMore data for this Ligand-Target Pair
TargetD(2) dopamine receptor(Homo sapiens (Human))
Institute Of Nuclear Chemistry Johannes Gutenberg-University Mainz
Curated by ChEMBL
Institute Of Nuclear Chemistry Johannes Gutenberg-University Mainz
Curated by ChEMBL
Affinity DataKi: 62nMAssay Description:Binding affinity to dopamine D2 receptor (unknown origin) by competitive binding experimentMore data for this Ligand-Target Pair
Affinity DataKi: 62nMAssay Description:Displacement of [3H]-halopridol from rat striatum D2 receptorsMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1A(Homo sapiens (Human))
Institute Of Nuclear Chemistry Johannes Gutenberg-University Mainz
Curated by ChEMBL
Institute Of Nuclear Chemistry Johannes Gutenberg-University Mainz
Curated by ChEMBL
Affinity DataKi: 1.57E+3nMAssay Description:Binding affinity to 5-HT1A receptor (unknown origin) by competitive binding experimentMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Antagonist activity at recombinant human 5-HT2A expressed in human U2OS cells by pathhunter beta-arrestin assayMore data for this Ligand-Target Pair