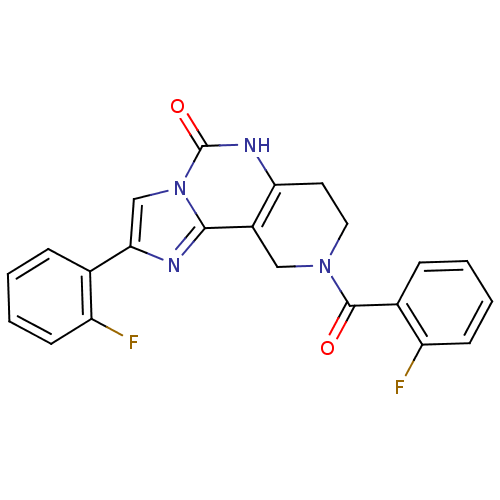
BDBM50120773 8-(2-Fluoro-benzoyl)-2-(2-fluoro-phenyl)-6,7,8,9-tetrahydro-5H-1,3a,5,8-tetraaza-cyclopenta[a]naphthalen-4-one::CHEMBL146302
SMILES Fc1ccccc1C(=O)N1CCc2[nH]c(=O)n3cc(nc3c2C1)-c1ccccc1F
InChI Key InChIKey=NHPMGBFDAPCAMB-UHFFFAOYSA-N
Data 4 EC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50120773
Found 4 hits for monomerid = 50120773
TargetGamma-aminobutyric acid receptor subunit alpha-5(Homo sapiens (Human))
Neurogen
Curated by ChEMBL
Neurogen
Curated by ChEMBL
Affinity DataEC50: 346nMAssay Description:Effective concentration against Gamma-aminobutyric acid A receptor, alpha 5More data for this Ligand-Target Pair
TargetGamma-aminobutyric acid receptor subunit alpha-3(Homo sapiens (Human))
Neurogen
Curated by ChEMBL
Neurogen
Curated by ChEMBL
Affinity DataEC50: 242nMAssay Description:Effective concentration against gamma-aminobutyric acid (GABA) A receptor, alpha 3More data for this Ligand-Target Pair
TargetGamma-aminobutyric acid receptor subunit alpha-2(Homo sapiens (Human))
Neurogen
Curated by ChEMBL
Neurogen
Curated by ChEMBL
Affinity DataEC50: 256nMAssay Description:Effective concentration against gamma-aminobutyric acid (GABA) A receptor, alpha 2More data for this Ligand-Target Pair
TargetGamma-aminobutyric acid receptor subunit alpha-1(Homo sapiens (Human))
Neurogen
Curated by ChEMBL
Neurogen
Curated by ChEMBL
Affinity DataEC50: 93nMAssay Description:Effective concentration against gamma-aminobutyric acid (GABA) A receptor, alpha 1More data for this Ligand-Target Pair