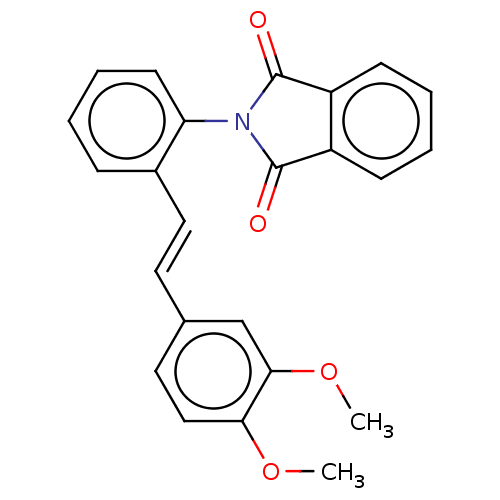
BDBM50122619 CHEMBL3623107
SMILES COc1ccc(\C=C\c2ccccc2N2C(=O)c3ccccc3C2=O)cc1OC
InChI Key InChIKey=MKFOSSOUEAENMG-ACCUITESSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50122619
Found 4 hits for monomerid = 50122619
Affinity DataIC50: 3.30E+3nMAssay Description:Antagonist activity at human LXR-alpha transfected in HEK293 cells after 16 hrs by luciferase reporter gene assay in presence of 0.3 uM N-(4-(1,1,1,3...More data for this Ligand-Target Pair
TargetOxysterols receptor LXR-alpha/LXR-beta(Homo sapiens (Human))
The University Of Tokyo
Curated by ChEMBL
The University Of Tokyo
Curated by ChEMBL
Affinity DataIC50: 1.50E+3nMAssay Description:Transrepression activity of LXR in human THP1 cells assessed as inhibition of LPS-induced IL-6 level after 18 hrs by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+3nMAssay Description:Binding affinity to LXR-beta ligand binding domain (unknown origin) by TR-FRET assay in presence of 0.1 uM agonist N-(4-(1,1,1,3,3,3-hexafluoro-2-hyd...More data for this Ligand-Target Pair
Affinity DataIC50: 4.30E+3nMAssay Description:Antagonist activity at human LXR-beta transfected in HEK293 cells after 16 hrs by luciferase reporter gene assay in presence of 0.1 uM N-(4-(1,1,1,3,...More data for this Ligand-Target Pair