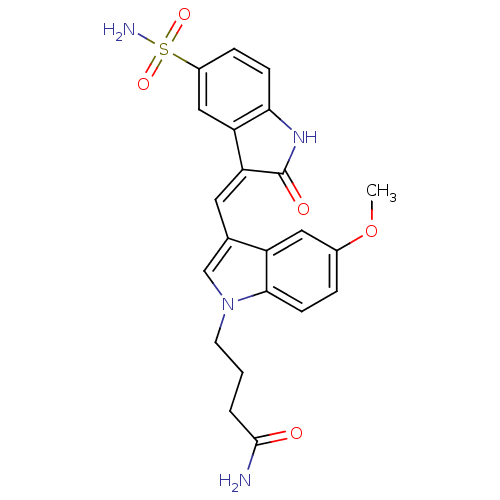
BDBM50132443 4-{5-Methoxy-3-[2-oxo-5-sulfamoyl-1,2-dihydro-indol-(3Z)-ylidenemethyl]-indol-1-yl}-butyramide::CHEMBL431645
SMILES COc1ccc2n(CCCC(N)=O)cc(\C=C3/C(=O)Nc4ccc(cc34)S(N)(=O)=O)c2c1
InChI Key InChIKey=YJHSHAAGDWUFBT-NVMNQCDNSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50132443
Found 4 hits for monomerid = 50132443
Affinity DataIC50: 12nMAssay Description:Inhibitory activity against human Syk protein tyrosine kinase expressed in yeast Klyveromyces lactisMore data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+4nMAssay Description:Inhibition of SYK in rat RBL2H3 cells assessed as reduction in DNP-BSA-stimulated Fcepsilon receptor 1-mediated 5-HT releaseMore data for this Ligand-Target Pair
TargetLow affinity immunoglobulin epsilon Fc receptor(Homo sapiens (Human))
Aventis Pharmaceuticals
Curated by ChEMBL
Aventis Pharmaceuticals
Curated by ChEMBL
Affinity DataEC50: >1.00E+4nMAssay Description:Effect on IgE/Fcepsilon RI triggered rat basophil cell (RBL-2H3) degranulation assessed by measuring the amount of 5-HT releaseMore data for this Ligand-Target Pair