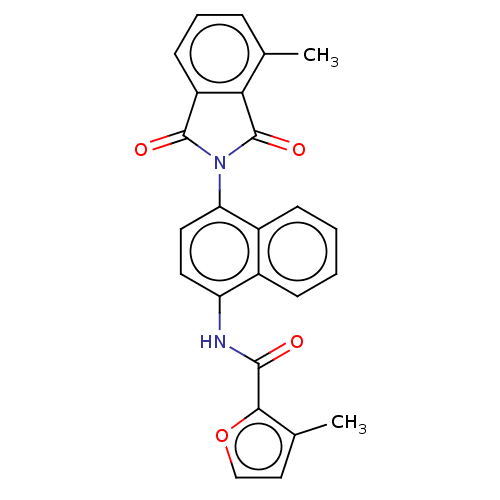
BDBM50132800 CHEMBL3634442
SMILES Cc1ccoc1C(=O)Nc1ccc(N2C(=O)c3cccc(C)c3C2=O)c2ccccc12
InChI Key InChIKey=WEXKYZXMDCSALY-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50132800
Found 3 hits for monomerid = 50132800
TargetMetabotropic glutamate receptor 1(Homo sapiens (Human))
Vanderbilt University Medical Center
Curated by ChEMBL
Vanderbilt University Medical Center
Curated by ChEMBL
Affinity DataEC50: 16nMAssay Description:Displacement of [3H]batrachotoxin from sodium channel in rat brainMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 4(Homo sapiens (Human))
Vanderbilt University Medical Center
Curated by ChEMBL
Vanderbilt University Medical Center
Curated by ChEMBL
Affinity DataEC50: 429nMAssay Description:Positive allosteric modulation of human mGluR4 by calcium mobilization assayMore data for this Ligand-Target Pair
TargetMetabotropic glutamate receptor 1(Homo sapiens (Human))
Vanderbilt University Medical Center
Curated by ChEMBL
Vanderbilt University Medical Center
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Positive allosteric modulation of human mGluR1 by calcium mobilization assayMore data for this Ligand-Target Pair