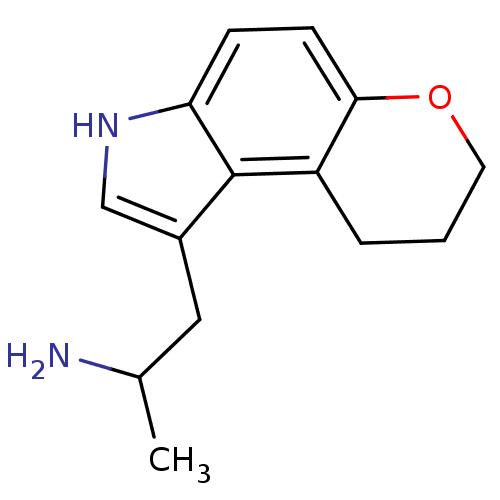
BDBM50133229 1-Methyl-2-(3,7,8,9-tetrahydro-pyrano[3,2-e]indol-1-yl)-ethylamine::CHEMBL133868
SMILES CC(N)Cc1c[nH]c2ccc3OCCCc3c12
InChI Key InChIKey=VVHJUSGIUWQPIT-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50133229
Found 4 hits for monomerid = 50133229
Affinity DataIC50: 1.09E+4nMAssay Description:In vitro inhibitory concentration required against [3H]8-OH-DPAT binding to cloned human 5-hydroxytryptamine 1A receptorMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibitory concentration required against Alpha-2A adrenergic receptor using [3H]clonidine radioligandMore data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:In vitro inhibition of [125I]DOI binding to 5-hydroxytryptamine 2A receptor in rat cerebral cortexMore data for this Ligand-Target Pair
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibitory concentration required against Alpha-2C adrenergic receptor using [3H]clonidine radioligandMore data for this Ligand-Target Pair