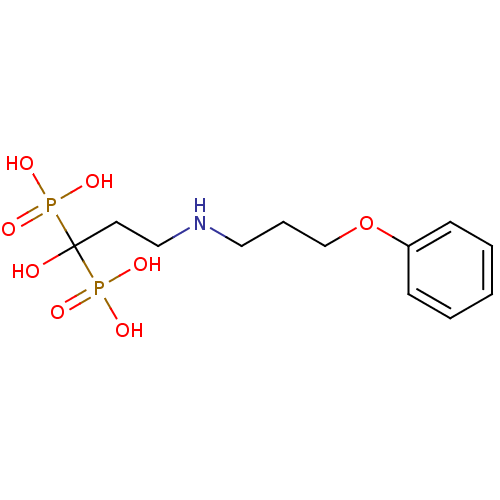
BDBM50135831 CHEMBL316844::[1-Hydroxy-3-(3-phenoxy-propylamino)-1-phosphono-propyl]-phosphonic acid
SMILES OC(CCNCCCOc1ccccc1)(P(O)(O)=O)P(O)(O)=O
InChI Key InChIKey=KZFMRKCPGOONMR-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50135831
Found 4 hits for monomerid = 50135831
TargetFarnesyl pyrophosphate synthase(Homo sapiens (Human))
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataKi: 43nMAssay Description:Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligandMore data for this Ligand-Target Pair
TargetFarnesyl pyrophosphate synthase(Homo sapiens (Human))
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataIC50: 447nMAssay Description:Inhibitory activity against farnesyl Pyrophosphate Synthase expressed as #NAME? (M)More data for this Ligand-Target Pair
TargetFarnesyl pyrophosphate synthase(Homo sapiens (Human))
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataIC50: 3.30nMAssay Description:Negative logarithm of inhibitory concentration against bone resorptionMore data for this Ligand-Target Pair
TargetFarnesyl pyrophosphate synthase(Homo sapiens (Human))
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
University Of Illinois At Urbana-Champaign
Curated by ChEMBL
Affinity DataIC50: 450nMAssay Description:Inhibitory activity against farnesyl Pyrophosphate Synthase was determinedMore data for this Ligand-Target Pair