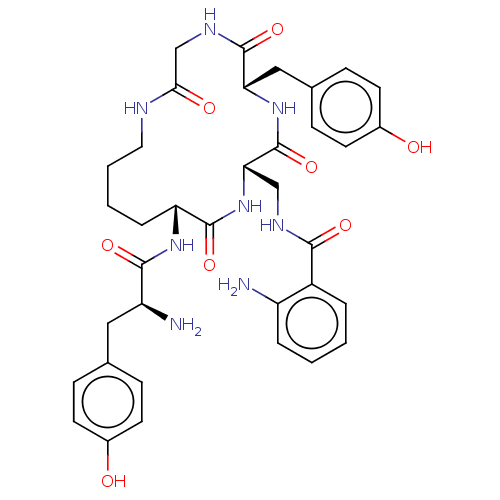
BDBM50140042 CHEMBL3763495
SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1CCCCNC(=O)CNC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CNC(=O)c2ccccc2N)NC1=O
InChI Key InChIKey=STLIVDBKTZZSIE-XJYHXZFBSA-N
Data 3 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50140042
Found 3 hits for monomerid = 50140042
TargetMu-type opioid receptor(Rattus norvegicus (rat))
Torrey Pines Institute For Molecular Studies
Curated by ChEMBL
Torrey Pines Institute For Molecular Studies
Curated by ChEMBL
Affinity DataKi: 14nMAssay Description:Displacement of [3H]DAMGO from rat cortical MOR by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetDelta-type opioid receptor(Rattus norvegicus (rat))
Torrey Pines Institute For Molecular Studies
Curated by ChEMBL
Torrey Pines Institute For Molecular Studies
Curated by ChEMBL
Affinity DataKi: 865nMAssay Description:Displacement of [3H]DPDPE from rat cortical DOR after 2.5 hrs by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Cavia porcellus (domestic guinea pig))
Torrey Pines Institute For Molecular Studies
Curated by ChEMBL
Torrey Pines Institute For Molecular Studies
Curated by ChEMBL
Affinity DataKi: 3.23E+3nMAssay Description:Displacement of [3H]U69,593 from guinea pig KOR after 2 hrs by liquid scintillation counting methodMore data for this Ligand-Target Pair