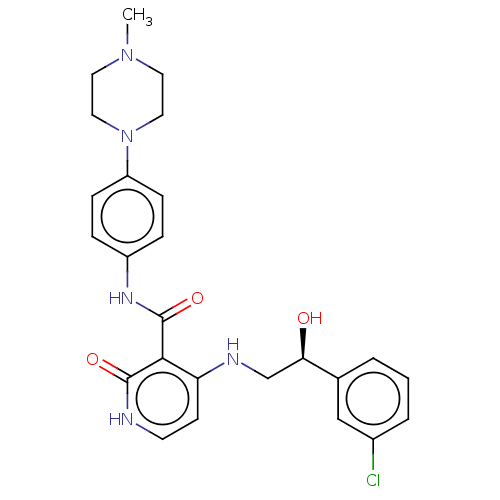
BDBM50157333 CHEMBL3781433
SMILES CN1CCN(CC1)c1ccc(NC(=O)c2c(NC[C@@H](O)c3cccc(Cl)c3)cc[nH]c2=O)cc1
InChI Key InChIKey=TUKPPSIVXDZORW-JOCHJYFZSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50157333
Found 3 hits for monomerid = 50157333
Affinity DataKi: 18nMAssay Description:Inhibition of human N-terminal GST-tagged EGFR catalytic domain (669 to 1210 residues) expressed in baculovirus expression system using Fl-EEPLYWSFPA...More data for this Ligand-Target Pair
Affinity DataKi: 65nMAssay Description:Inhibition of human EGFR catalytic domain (668 to 1210 residues) T790M/L858R double mutant using Fl-EEPLYWSFPAKKK-CONH2 peptide substrate preincubate...More data for this Ligand-Target Pair
Affinity DataEC50: 412nMAssay Description:Inhibition of EGFR T790M/L858R double mutant phosphorylation in human H1975 cells preincubated for 1 hr followed by EGF stimulation for 8 mins by ele...More data for this Ligand-Target Pair