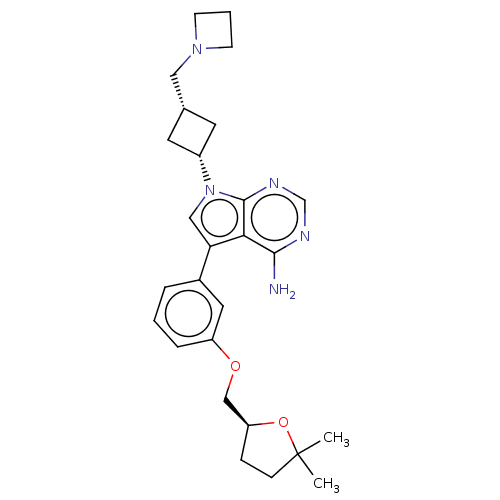
BDBM50157878 CHEMBL3786567
SMILES CC1(C)CC[C@@H](COc2cccc(c2)-c2cn([C@@H]3C[C@H](CN4CCC4)C3)c3ncnc(N)c23)O1
InChI Key InChIKey=BCTIPSBBPQBXAG-DWLFOUALSA-N
Data 10 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 50157878
Found 10 hits for monomerid = 50157878
TargetTyrosine-protein kinase Lck(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 7.40E+3nMAssay Description:Tested for neuronal nicotinic acetylcholine receptor (nAChR) binding in a whole rat brain preparation using [3H]-cystine as the radioligand.More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of Her1 (unknown origin)More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: >9.00E+3nMAssay Description:Inhibition of PI3K-beta (unknown origin)More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: >9.00E+3nMAssay Description:Inhibition of PI3K-alpha (unknown origin)More data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of KDR (unknown origin)More data for this Ligand-Target Pair
TargetInsulin receptor(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibition of Ins receptor (unknown origin)More data for this Ligand-Target Pair
TargetEphrin type-B receptor 4(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 690nMAssay Description:Inhibition of EphB4 receptor (unknown origin)More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 970nMAssay Description:Inhibition of Ret (unknown origin)More data for this Ligand-Target Pair
TargetTyrosine-protein kinase JAK1(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of Jak1 (unknown origin)More data for this Ligand-Target Pair
TargetInsulin-like growth factor 1 receptor(Homo sapiens (Human))
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Novartis Institutes For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Inhibition of IGF-1 receptor (unknown origin)More data for this Ligand-Target Pair