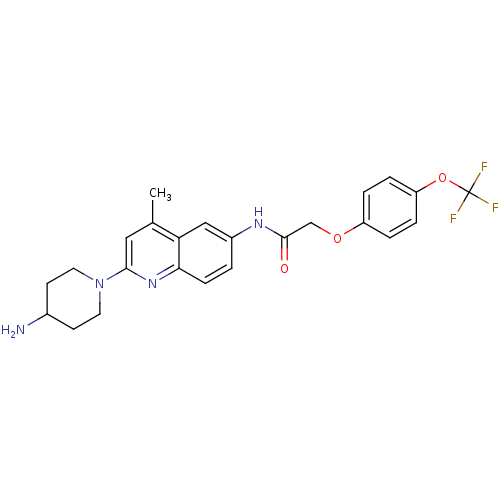
BDBM50172425 CHEMBL197026::N-[2-(4-Amino-piperidin-1-yl)-4-methyl-quinolin-6-yl]-2-(4-trifluoromethoxy-phenoxy)-acetamide
SMILES Cc1cc(nc2ccc(NC(=O)COc3ccc(OC(F)(F)F)cc3)cc12)N1CCC(N)CC1
InChI Key InChIKey=BEVTZBDUQNCQFB-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50172425
Found 4 hits for monomerid = 50172425
Affinity DataIC50: 4.70nMAssay Description:Displacement of 15 pM [125I]-MCH from human MCH2R expressed in CHO-K1 in whole-cell binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Displacement of 15 pM [125I]-MCH from human MCH1R expressed in CHO-K1 in whole-cell binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Inhibitory concentration against 10 nM MCH-induced IP3 accumulation in CHO-K1 cells expressing human MCH1R after incubation with [3H]-myo-inositolMore data for this Ligand-Target Pair
Affinity DataIC50: 3.5nMAssay Description:Displacement of 150p M [125I]-MCH from human MCH1R expressed in CHO-K1 cellsMore data for this Ligand-Target Pair