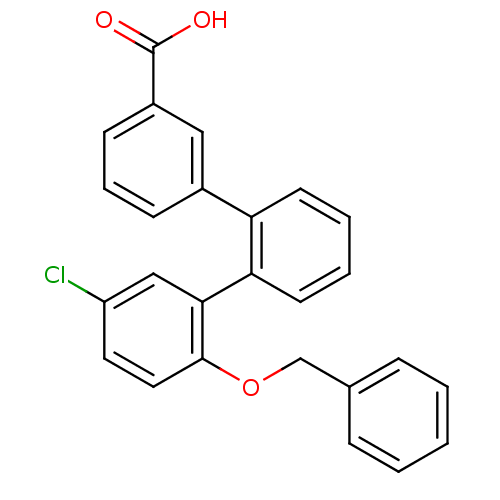
BDBM50183182 2''-benzyloxy-5''-chloro-[1,1';2',1'']terphenyl-3-carboxylic acid::2-Benzyloxy-5-chloro-[1,1';2',1'']terphenyl-3''-carboxylic acid::CHEMBL207174
SMILES OC(=O)c1cccc(c1)-c1ccccc1-c1cc(Cl)ccc1OCc1ccccc1
InChI Key InChIKey=LVEWBMDPAYFEFT-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50183182
Found 4 hits for monomerid = 50183182
Affinity DataKi: 31.6nMAssay Description:Antagonist activity at EP1 receptor assessed as calcium mobilization by FLIPR assayMore data for this Ligand-Target Pair
Affinity DataIC50: 50.1nMAssay Description:Displacement of [3H]PGE2 from EP1 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 3.90E+3nMAssay Description:Inhibition of CYP450 2C9More data for this Ligand-Target Pair
Affinity DataIC50: 50.1nMAssay Description:Binding affinity to EP1 receptorMore data for this Ligand-Target Pair