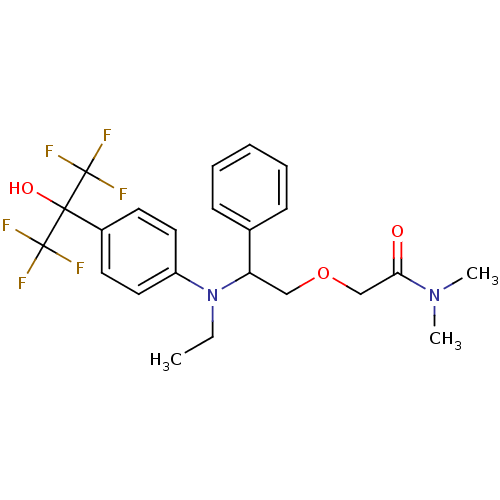
BDBM50192114 2-(2-(ethyl(4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)phenyl)amino)-2-phenylethoxy)-N,N-dimethylacetamide::CHEMBL215129
SMILES CCN(C(COCC(=O)N(C)C)c1ccccc1)c1ccc(cc1)C(O)(C(F)(F)F)C(F)(F)F
InChI Key InChIKey=HSXPHPZTVBAFKE-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50192114
Found 4 hits for monomerid = 50192114
Affinity DataIC50: 500nMAssay Description:Binding affinity to LXRbeta by radioligand displacement assayMore data for this Ligand-Target Pair
Affinity DataEC50: 800nMAssay Description:Transactivation of LXRalpha by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 260nMAssay Description:Transactivation of LXRbeta by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 900nMAssay Description:Binding affinity to LXRalpha by radioligand displacement assayMore data for this Ligand-Target Pair